
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
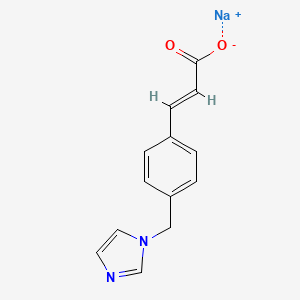

1. 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-2-propenoic Acid
2. 4-(1-imidazoylmethyl)cinnamic Acid
3. Oky 046
4. Oky-046
5. Ozagrel
6. Ozagrel, Monohydrochloride
7. Ozagrel, Monohydrochloride, (e)-isomer
8. Sodium Ozagrel
1. 130952-46-4
2. 189224-26-8
3. Cataclot
4. Xanbon
5. Sodium Ozagrel
6. Ozagrel (sodium)
7. Athrombone
8. Kadenin
9. Ozagrel Sodium [jan]
10. Ozagrel Sodium Salt [mi]
11. Sodium (e)-3-(4-((1h-imidazol-1-yl)methyl)phenyl)acrylate
12. Ozagrel Sodium [who-dd]
13. Sodium 3-(4-((1h-imidazol-1-yl)methyl)phenyl)acrylate
14. Kw-01
15. 4x5577n3et
16. 189224-26-8 (sodium)
17. 2-propenoic Acid, 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-, Sodium Salt, (2e)-
18. 2-propenoic Acid, 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-, Sodium Salt, (e)-
19. 2-propenoic Acid, 3-[4-(1h-imidazol-1-ylmethyl)phenyl]-, Sodium Salt (1:1)
20. 2-propenoic Acid, 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-, Sodium Salt (1:1), (2e)-
21. Sodium;(e)-3-[4-(imidazol-1-ylmethyl)phenyl]prop-2-enoate
22. Unii-4x5577n3et
23. Xanbons
24. Ozapen (tn)
25. Ozagrel Sodium (jp17)
26. Schembl3093220
27. Sodium,3-[4-(imidazol-1-ylmethyl)phenyl]prop-2-enoate
28. Hy-b0428a
29. Amy3367
30. Dtxsid50172327
31. Hms3887a15
32. Bcp12393
33. Ex-a5745
34. Akos015895382
35. Akos015967214
36. Akos025149474
37. Ccg-266963
38. Cs-3808
39. Ac-15516
40. As-12150
41. Gd95350000
42. D01684
43. A912378
44. Oky-046 Sodium;oky 046 Sodium;oky046 Sodium
45. Q27260625
46. Sodium(e)-3-(4-((1h-imidazol-1-yl)methyl)phenyl)acrylate
| Molecular Weight | 250.23 g/mol |
|---|---|
| Molecular Formula | C13H11N2NaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 250.07182188 g/mol |
| Monoisotopic Mass | 250.07182188 g/mol |
| Topological Polar Surface Area | 58 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 289 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?