
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
Canada

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
Weekly News Recap #Phispers
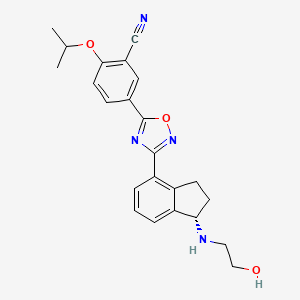

1. Rpc1063
1. 1306760-87-1
2. Rpc1063
3. Rpc-1063
4. Ozanimod (rpc1063)
5. (s)-5-(3-(1-((2-hydroxyethyl)amino)-2,3-dihydro-1h-inden-4-yl)-1,2,4-oxadiazol-5-yl)-2-isopropoxybenzonitrile
6. Z80293urpv
7. Zeposia
8. Unii-z80293urpv
9. Benzonitrile, 5-(3-((1s)-2,3-dihydro-1-((2-hydroxyethyl)amino)-1h-inden-4-yl)-1,2,4-oxadiazol-5-yl)-2-(1-methylethoxy)-
10. Ozanimod [inn]
11. 5-[3-[(1~{s})-1-(2-hydroxyethylamino)-2,3-dihydro-1~{h}-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-propan-2-yloxy-benzenecarbonitrile
12. Benzonitrile, 5-[3-[(1s)-2,3-dihydro-1-[(2-hydroxyethyl)amino]-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-(1-methylethoxy)-
13. Ozanimod [usan:inn]
14. 5-[3-[(1s)-1-(2-hydroxyethylamino)-2,3-dihydro-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-propan-2-yloxybenzonitrile
15. Rpc 1063
16. Ozanimod [usan]
17. Ozanimod; Rpc1063
18. Ozanimod (usan/inn)
19. Ozanimod [mi]
20. Ozanimod [who-dd]
21. Gtpl8709
22. Schembl2195490
23. Chembl3707247
24. Amy3373
25. Dtxsid501026488
26. Bcp16513
27. Ex-a1316
28. Rpc1063:rpc-1063
29. Bdbm50507186
30. Mfcd28386168
31. S7952
32. Akos026674086
33. Zinc116109867
34. Ccg-268695
35. Cs-5070
36. Db12612
37. Ac-29883
38. As-75063
39. Hy-12288
40. J3.612.016i
41. D10968
42. P14657
43. Q21098986
44. 5-{3-[(1s)-1-[(2-hydroxyethyl)amino]-2,3-dihydro-1h-inden-4-yl]-1,2,4-oxadiazol-5-yl}-2-(propan-2-yloxy)benzonitrile
45. Jeu
| Molecular Weight | 404.5 g/mol |
|---|---|
| Molecular Formula | C23H24N4O3 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 404.18484064 g/mol |
| Monoisotopic Mass | 404.18484064 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 609 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ozanimod is indicated for adults in the treatment of relapsing forms of MS, which may include relapsing-remitting disease, clinically isolated syndrome, and active secondary progressive MS.
FDA Label
Multiple sclerosis
- Zeposia is indicated for the treatment of adult patients with relapsing remitting multiple sclerosis (RRMS) with active disease as defined by clinical or imaging features.
Ulcerative colitis
- Zeposia is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response, or were intolerant to either conventional therapy or a biologic agent.
Ozanimod reduces circulating lymphocytes that cause the neuroinflammation associated with MS, reducing debilitating symptoms and, possibly, disease progression. During clinical trials, ozanimod reduced MS-associated brain volume loss in several regions. Ozanimod causes the sequestration of peripheral lymphocytes, reducing circulating lymphocytes in the gastrointestinal tract.
Sphingosine 1 Phosphate Receptor Modulators
Agents that affect the function of G-protein coupled SPHINGOSINE 1-PHOSPHATE RECEPTORS. Their binding to the receptors blocks lymphocyte migration and are often used as IMMUNOSUPPRESSANTS. (See all compounds classified as Sphingosine 1 Phosphate Receptor Modulators.)
L04AA38
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA38 - Ozanimod
Absorption
Ozanimod is absorbed in the gastrointestinal tract after oral administration. The Cmax of ozanimod is 0.244 ng/mL and is achieved at 6 to 8 hours after administration, reaching steady-state at about 102 hours after administration. The AUC is 4.46 ng*h/mL. Its delayed absorption reduces effects that may occur after the first dose, such as heart rate changes. The peak plasma concentration of ozanimod is low due to a high volume of distribution.
Route of Elimination
The kidneys are not a major source of elimination for ozanimod. After a 0.92 mg dose of radiolabeled ozanimod was administered, about 26% of the labeled drug was accounted for in the urine and 37 % in the feces, mainly in the form of inactive metabolites.
Volume of Distribution
The average volume of distribution of ozanimod is 5590L. Another reference mentions a volume of distribution ranging from 73-101 L/kg. This drug crosses the blood-brain barrier.
Clearance
The mean apparent oral clearance of ozanimod, according to prescribing information, is 192 L/h. Another reference indicates an oral clearance of 233 L/h.
Ozanimod has two major active metabolites CC112273 and CC1084037 and minor active metabolites such as RP101988, RP101075, and RP101509, which target the S1P1 and S1P5 receptors. The enzymes involved in the metabolism of ozanimod include ALDH/ADH, NAT-2, Monoamine Oxidase B, and AKR 1C1/1C2. After metabolism, ozanimod (6%), CC112273 (73%), and CC1084037 (15%) are accounted for in the circulation.
The half-life of ozanimod ranges from 17-21 hours.
Sphingosine1phosphate (S1P) is an important phospholipid that binds to various Gproteincoupled receptor subtypes, which can be identified as S1P15R. S1P and the receptors it binds to perform regular functions in the immune, cardiovascular, pulmonary, and nervous system. S1P can be expressed ubiquitously, playing an important role in regulating inflammation. S1P1R, S1P2R, and S1P3R receptors can be found in the cardiovascular, immune, and central nervous systems. S1P4R is found on lymphocytic and hematopoietic cells, while S1P5R expression is found only on the spleen (on natural killer cells) or in the central nervous system. Ozanimod is a selective modulator of S1P receptors and binds to S1P1R and S1P5R subtypes. The mechanism of action of ozanimod is not fully understood, but this drug likely reduces the migration of lymphocytes that usually aggravate the inflammation associated with MS.
 Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37597
Submission : 2022-10-21
Status : Active
Type : II

NDC Package Code : 52076-6266
Start Marketing Date : 2020-12-11
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-11-10
Pay. Date : 2023-09-15
DMF Number : 38622
Submission : 2023-09-22
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-01-22
Pay. Date : 2023-11-17
DMF Number : 38668
Submission : 2023-11-23
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37015
Submission : 2022-03-31
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8531
Submission : 1990-04-17
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8462
Submission : 1990-02-22
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
Chunghwa provides cost-effective APIs & advanced intermediates with complete DMF or COS, ensuring quality & reliable production.
About the Company : Chunghwa Chemical Synthesis & Biotech Co. Ltd. (CCSB) offers global customers cost-effective APIs with speed, supported by our well-trained staff. As the first company in Southeast...
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
About the Company : Polpharma API. European CDMO partner and API manufacturer since 1951. Poland-based CDMO and API producer, delivering products to pharmaceutical companies worldwide. Aiming at the c...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
About the Company : Egis is a member of the Servier Group. Egis’ products are manufactured at 3 production sites in Hungary, which are certified by EMA,FDA, ANVISA, PMDA ,KFDA. Egis sells its produc...
 Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
Faran Shimi: Leading producer of high-quality APIs & alkaloid opiates, serving major pharmaceutical companies across the Middle East.
About the Company : Faran Shimi Pharmaceutical Company, established in 2001 and affiliated with Golrang Pharmaceutical Investment Co, manufactures high-quality Active Pharmaceutical Ingredients (APIs)...
About the Company : More than 35 years of dedication to quality, service and pursuit of excellence, CHEMO was founded by Hugo Sigman, M.D., and Silvia Gold, Biochemist, in Spain (Barcelona), in 1977, ...

About the Company : Maithri Drugs is one of India's fast-growing pharmaceutical companies. Maithri's strategic focus is on active pharma ingredients (APIs). The company is widely recognized for its ex...

About the Company : Mankind Pharma, came into existence in 1986. In 1991, the company was formed into a legal corporation. However, it actively started working as a fully integrated pharmaceutical com...

About the Company : Established in the early 90’s SCL offers a wide range of Active Pharmaceutical Ingredients and Intermediates to its customers worldwide. SCL’s products are exported to Europe, ...

About the Company : Suzhou Biosyntech is an R&D driven supplier for the Pharma market, the major business is on API and intermediates. Biosyntech has the ability to provide service from R&D developmen...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Regulatory Info :
Registration Country : Iran
Brand Name : Wellosia
Dosage Form : Capsule
Dosage Strength : 0.23MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Iran
Regulatory Info :
Registration Country : Iran
Brand Name : Wellosia
Dosage Form : Capsule
Dosage Strength : 0.46MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Iran
Regulatory Info :
Registration Country : Iran
Brand Name : Wellosia
Dosage Form : Capsule
Dosage Strength : 0.92MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Iran
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ZEPOSIA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 0.23MG BASE
Packaging :
Approval Date : 2020-03-25
Application Number : 209899
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ZEPOSIA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 0.46MG BASE
Packaging :
Approval Date : 2020-03-25
Application Number : 209899
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ZEPOSIA
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 0.92MG BASE
Packaging :
Approval Date : 2020-03-25
Application Number : 209899
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Zeposia
Dosage Form : Caps
Dosage Strength : 0.92mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Zeposia
Dosage Form :
Dosage Strength :
Packaging : 28
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Zeposia
Dosage Form :
Dosage Strength :
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Zeposia
Dosage Form :
Dosage Strength :
Packaging : 1
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Reply
26 Jul 2022
Reply
26 Sep 2020
Reply
12 Jun 2020
Reply
10 Jun 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?