
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
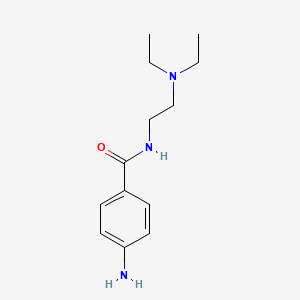

1. Amide, Procaine
2. Apo-procainamide
3. Biocoryl
4. Hydrochloride, Procainamide
5. Novocainamide
6. Novocamid
7. Procainamide Hydrochloride
8. Procaine Amide
9. Procamide
10. Procan
11. Procan Sr
12. Procanbid
13. Pronestyl
14. Rhythmin
1. 51-06-9
2. Novocainamide
3. Biocoryl
4. Novocamid
5. 4-amino-n-[2-(diethylamino)ethyl]benzamide
6. Procaine Amide
7. 4-amino-n-(2-diethylaminoethyl)benzamide
8. Pronestyl
9. Novocainamid
10. Procamide
11. Novocaine Amide
12. Procainamida
13. Procainamidum
14. Procan
15. Procapan (free Base)
16. P-aminobenzoic Diethylaminoethylamide
17. P-amino-n-(2-diethylaminoethyl)benzamide
18. Pronestyl-sr
19. 4-amino-n-(2-(diethylamino)ethyl)benzamide
20. Nsc 27461
21. Benzamide, 4-amino-n-(2-(diethylamino)ethyl)-
22. Procainamide (inn)
23. Chebi:8428
24. 2-diethylaminoethylamid Kyseliny P-aminobenzoove
25. Nsc-27461
26. Benzamide, 4-amino-n-[2-(diethylamino)ethyl]-
27. Sp 100 (pharmaceutical)
28. L39wtc366d
29. Benzamide, P-amino-n-(2-(diethylamino)ethyl)-
30. Benzamide, P-amino-n-[2-(diethylamino)ethyl]-
31. Ncgc00015859-07
32. Rhythmin
33. 4-amino-n-(2-diethylaminoethyl) Benzamide
34. Dsstox_cid_3512
35. Procainamide [inn]
36. Procanbid; Sp 100; Sp 100 (pharmaceutical)
37. Dsstox_rid_77059
38. Dsstox_gsid_23512
39. Procainamide [inn:ban]
40. Procainamidum [inn-latin]
41. Procainamida [inn-spanish]
42. Cas-51-06-9
43. Hsdb 3170
44. Cas-614-39-1
45. Einecs 200-078-8
46. Brn 2214285
47. Unii-l39wtc366d
48. 2-diethylaminoethylamid Kyseliny P-aminobenzoove [czech]
49. Spectrum_000836
50. Maybridge1_004389
51. Prestwick0_000337
52. Prestwick1_000337
53. Prestwick2_000337
54. Prestwick3_000337
55. Spectrum2_001295
56. Spectrum3_000555
57. Spectrum4_000487
58. Spectrum5_000986
59. Lopac-p-9391
60. Chembl640
61. Epitope Id:135397
62. Procainamide [hsdb]
63. Cambridge Id 5144127
64. Procainamide [vandf]
65. Lopac0_000995
66. Schembl15914
67. Bspbio_000373
68. Bspbio_001463
69. Bspbio_002229
70. Cbdive_003757
71. Kbiogr_000183
72. Kbiogr_000973
73. Kbioss_000183
74. Kbioss_001316
75. 4-14-00-01154 (beilstein Handbook Reference)
76. Cid_66068
77. Bidd:gt0579
78. Divk1c_000931
79. Procainamide [who-dd]
80. Spbio_001329
81. Spbio_002294
82. Wln: Zr Dvm2n2&2
83. Bpbio1_000411
84. Gtpl4811
85. Dtxsid7023512
86. Bdbm39344
87. Hms553p13
88. Hy-a0084a
89. Kbio1_000931
90. Kbio2_000183
91. Kbio2_001316
92. Kbio2_002751
93. Kbio2_003884
94. Kbio2_005319
95. Kbio2_006452
96. Kbio3_000365
97. Kbio3_000366
98. Kbio3_001729
99. Ninds_000931
100. Bio1_000391
101. Bio1_000880
102. Bio1_001369
103. Bio2_000183
104. Bio2_000663
105. Hms1361j05
106. Hms1791j05
107. Hms1989j05
108. Hms2089e13
109. Hms3402j05
110. Nsc27461
111. Zinc1530756
112. Tox21_110246
113. Mfcd00066880
114. Stk367963
115. Akos000271131
116. Tox21_110246_1
117. 4-amino-n-(diethylaminoethyl)benzamide
118. Ccg-205075
119. Cs-w009100
120. Db01035
121. Fs-5697
122. Sdccgsbi-0050968.p005
123. Idi1_000931
124. Idi1_033933
125. Smp1_000055
126. Ncgc00015859-01
127. Ncgc00015859-02
128. Ncgc00015859-03
129. Ncgc00015859-04
130. Ncgc00015859-05
131. Ncgc00015859-06
132. Ncgc00015859-08
133. Ncgc00015859-09
134. Ncgc00015859-10
135. Ncgc00015859-11
136. Ncgc00015859-14
137. Ncgc00015859-16
138. Ncgc00015859-18
139. Ncgc00015859-23
140. Ncgc00024323-03
141. Ncgc00024323-04
142. Ncgc00024323-05
143. Ncgc00024323-06
144. N-(2-diethylaminoethyl) 4-aminobenzamide
145. Sbi-0050968.p004
146. 4-amino-n-(2-diethylamino-ethyl)-benzamide
147. Ab00053530
148. Bb 0216450
149. N1-[2-(diethylamino)ethyl]-4-aminobenzamide
150. 4-{n-[2-(diethylamino)ethyl]carbamoyl}aniline
151. 4-amino-n-[2-(diethylamino)ethyl]benzamide #
152. C07401
153. C75392
154. D08421
155. Ab00053530-13
156. Ab00053530-15
157. Ab00053530_16
158. 051p069
159. L001052
160. Q417597
161. N-(2-(diethylamino)ethyl)-4-aminobenzamide
162. Brd-k75089421-001-02-5
163. Brd-k75089421-003-04-7
164. Brd-k75089421-003-05-4
165. Brd-k75089421-003-15-3
166. F2173-1035
167. 4-azanyl-n-[2-(diethylamino)ethyl]benzamide;hydrochloride
| Molecular Weight | 235.33 g/mol |
|---|---|
| Molecular Formula | C13H21N3O |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 235.168462302 g/mol |
| Monoisotopic Mass | 235.168462302 g/mol |
| Topological Polar Surface Area | 58.4 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 221 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Arrhythmia Agents; Platelet Aggregation Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
PROCAINAMIDE IS USEFUL IN SUPPRESSING ARRHYTHMIAS OF VENTRICULAR ORIGIN, INCL VENTRICULAR EXTRASYSTOLES, PAROXYSMAL VENTRICULAR TACHYCARDIA, & VENTRICULAR FIBRILLATION. /HYDROCHLORIDE SALT/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 794
DRUG IS...EFFECTIVE AGAINST PAROXYSMAL ATRIAL TACHYCARDIA, ATRIAL FLUTTER, & ATRIAL TACHYCARDIA OR ATRIAL ECTOPIC SYSTOLES. IN CASES OF PAROXYSMAL ATRIAL TACHYCARDIA, OTHER MEASURES & AGENTS OF CHOICE SHOULD BE EMPLOYED BEFORE PROCAINAMIDE IS USED. /HYDROCHLORIDE SALT/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 794
PROCAINAMIDE HAS BEEN USED IN TREATMENT OF MYOTONIA, WHERE ITS EFFECTS RESEMBLE THOSE OF QUININE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 696
For more Therapeutic Uses (Complete) data for PROCAINAMIDE (8 total), please visit the HSDB record page.
...USED WITH CAUTION & MEDICATION MUST BE STOPPED IF QRS COMPLEX IS EXCESSIVELY WIDENED. PROCAINAMIDE IS USUALLY WELL TOLERATED. HOWEVER, IT HAS OCCASIONALLY CAUSED SERIOUS SIDE EFFECTS, & DEATHS HAVE RESULTED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
...PROCAINAMIDE CAN CAUSE UNTOWARD RESPONSES BY ITS ACTIONS ON ABNORMAL MYOCARDIUM OR AS RESULT OF CORRECTION OF ARRHYTHMIAS FOR WHICH DRUG IS ADMIN. ... PROCAINAMIDE...SHOULD NOT BE ADMIN WHEN COMPLETE A-V BLOCK IS PRESENT & SHOULD BE USED ONLY CAUTIOUSLY IN PRESENCE OF PARTIAL BLOCK BECAUSE OF DANGER OF ASYSTOLE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
CROSS SENSITIVITY TO PROCAINE & RELATED DRUGS SHOULD BE ANTICIPATED. ... /IT/...MUST BE GIVEN CAUTIOUSLY IF PT IS DIGITALIZED. /HCL SALT/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 794
FATAL AGRANULOCYTOSIS...REPORTED, & FREQUENT BLOOD EXAM DURING CHRONIC... THERAPY ARE ESSENTIAL. SYNDROME SIMILAR TO SYSTEMIC LUPUS ERYTHEMATOSUS IS COMMON REACTION TO CHRONIC ADMIN, & MAY NECESSITATE TERMINATION OF THERAPY...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 696
For more Drug Warnings (Complete) data for PROCAINAMIDE (9 total), please visit the HSDB record page.
For the treatment of life-threatening ventricular arrhythmias.
Procainamide is an agent indicated for production of local or regional anesthesia and in the treatment of ventricular tachycardia occurring during cardiac manipulation, such as surgery or catheterization, or which may occur during acute myocardial infarction, digitalis toxicity, or other cardiac diseases. The mode of action of the antiarrhythmic effect of Procainamide appears to be similar to that of procaine and quinidine. Ventricular excitability is depressed and the stimulation threshold of the ventricle is increased during diastole. The sinoatrial node is, however, unaffected.
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
C - Cardiovascular system
C01 - Cardiac therapy
C01B - Antiarrhythmics, class i and iii
C01BA - Antiarrhythmics, class ia
C01BA02 - Procainamide
Absorption
75 to 95%
Route of Elimination
Trace amounts may be excreted in the urine as free and conjugated p-aminobenzoic acid, 30 to 60 percent as unchanged PA, and 6 to 52 percent as the NAPA derivative.
Volume of Distribution
2 L/kg
PROCAINAMIDE IS RAPIDLY & ALMOST COMPLETELY ABSORBED FROM GI TRACT. WHEN... GIVEN ORALLY, ITS PLASMA CONCN BECOMES MAX IN ABOUT 60 MIN; AFTER IM ADMIN PEAK PLASMA CONCN ARE REACHED IN 15-60 MIN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
AT ORDINARY PLASMA CONCN, ONLY 15%...IS BOUND TO MACROMOLECULAR CONSTITUENTS OF PLASMA. CONCN OF DRUG IN MOST TISSUES EXCEPT BRAIN IS GREATER THAN THAT IN PLASMA. APPROX 60% OF DRUG IS EXCRETED BY KIDNEY. TWO TO 10%...IS RECOVERED IN URINE AS FREE & CONJUGATED P-AMINOBENZOIC ACID.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
CONSIDERABLE DIFFERENCES...OBSERVED IN PROCAINAMIDE & ALSO PROCAINAMIDE ETHOBROMIDE DISPOSITION. ETHOBROMIDE IS CLEARED RAPIDLY IN BILE OF RATS & RABBITS, BUT ONLY SLOWLY IN DOGS. 64% OF ORALLY DOSED PROCAINAMIDE IS VOIDED AS UNCHANGED...IN HUMAN URINE, WHEREAS IN RHESUS MONKEY IT IS ALMOST COMPLETELY METABOLIZED.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 149
RENAL, CARDIAC, OR HEPATIC IMPARIMENT...RESULTED IN PROLONGED PLASMA HALF-LIVES, & THERE IS EVIDENCE THAT PROCAINAMIDE MAY INHIBIT ITS OWN ELIMINATION AFTER MULTIPLE DOSING.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 447
For more Absorption, Distribution and Excretion (Complete) data for PROCAINAMIDE (7 total), please visit the HSDB record page.
Hepatic
DRUG IS RELATIVELY SLOWLY HYDROLYZED BY PLASMA ESTERASES.../PRC: AND BY MICROSOMAL ENZYMES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
PROCAINAMIDE...WAS METABOLIZED TO N-ACETYL DERIV FOLLOWING ORAL ADMIN TO MAN & RHESUS MONKEYS. ... TWO MAJOR METABOLITES WERE DETECTED IN MONKEY URINE, P-ACETAMIDOBENZOIC ACID & DE-ETHYLATED DERIV, P-ACETAMIDO-N-[2-(ETHYLAMINO)-ETHYL]BENZAMIDE.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 553
N-ACETYLPROCAINAMIDE IS AN ACTIVE METABOLITE OF PROCAINAMIDE.
LEE ET AL; ANTI-ARRHYTHMIC EFFICACY OF N-ACETYLPROCAINAMIDE IN PATIENTS WITH PREMATURE VENTRICULAR CONTRACTIONS; CLIN PHARMACOL THER 19(MAY) 508-514 (1976)
Procainamide has known human metabolites that include Acecainide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
~2.5-4.5 hours
PLASMA HALF-LIVES OF PARENT DRUG & ITS TWO MAJOR METABOLITES IN MAN IS BETWEEN 2 & 3 HR. ELIMINATION IS DIRECTLY RELATED TO CREATININE CLEARANCE, & PLASMA HALF-LIFE OF UNCHANGED DRUG IS CONSIDERABLY INCR IN CASES OF RENAL IMPAIRMENT.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 149
...BIOLOGICAL HALF-LIFE IS 3-4 HR...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
Procainamide is sodium channel blocker. It stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses thereby effecting local anesthetic action.
IV ADMIN...CAUSES FALL IN BLOOD PRESSURE; PERIPHERAL VASODILATATION PROBABLY CONTRIBUTES TO HYPOTENSIVE RESPONSE, BUT SYSTOLIC PRESSURE MAY BE REDUCED MORE THAN DIASTOLIC. ... CNS ACTIONS OF PROCAINAMIDE ARE NOT PROMINENT.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 695
Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?