
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
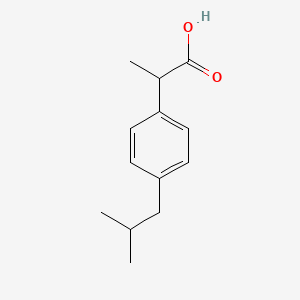

1. Advil
2. Alpha-methyl-4-(2-methylpropyl)benzeneacetic Acid
3. Benzeneacetic Acid, Alpha-methyl-4-(2-methylpropyl)- Trimethylsilyl Ester
4. Brufen
5. Ibumetin
6. Ibuprofen Zinc
7. Ibuprofen, (+-)-isomer
8. Ibuprofen, (r)-isomer
9. Ibuprofen, (s)-isomer
10. Ibuprofen, Aluminum Salt
11. Ibuprofen, Calcium Salt
12. Ibuprofen, Copper (2+) Salt
13. Ibuprofen, Magnesium Salt
14. Ibuprofen, Potassium Salt
15. Ibuprofen, Sodium Salt
16. Ibuprofen, Zinc Salt
17. Ibuprofen-zinc
18. Motrin
19. Nuprin
20. Rufen
21. Salprofen
22. Trauma Dolgit Gel
23. Trauma-dolgit Gel
1. 15687-27-1
2. 2-(4-isobutylphenyl)propanoic Acid
3. Motrin
4. Brufen
5. Advil
6. Nuprin
7. Nurofen
8. Medipren
9. Dolgit
10. Liptan
11. 2-[4-(2-methylpropyl)phenyl]propanoic Acid
12. Anflagen
13. Buburone
14. Butylenin
15. Ibumetin
16. Ibuprocin
17. Ebufac
18. Lamidon
19. Rufen
20. Mynosedin
21. Apsifen
22. Epobron
23. Haltran
24. Roidenin
25. Trendar
26. Adran
27. Andran
28. Bluton
29. Nobfen
30. Nobgen
31. Pediaprofen
32. Ibuprohm
33. Nobfelon
34. Brufort
35. Burana
36. Inabrin
37. Pantrop
38. Rebugen
39. Suspren
40. Tabalon
41. (rs)-ibuprofen
42. Anco
43. Urem
44. Ibu-slo
45. Brufanic
46. Napacetin
47. Ibufen
48. Ibuprin
49. Profen
50. Ibren
51. Midol
52. 4-isobutylhydratropic Acid
53. Cap-profen
54. Tab-profen
55. Ibu-tab
56. P-isobutylhydratropic Acid
57. Proartinal
58. Dibufen
59. Optifen
60. Ostofen
61. Panafen
62. Proflex
63. Quadrax
64. Uprofen
65. Carol
66. Dolgin
67. Cobo
68. (+-)-ibuprofen
69. (+/-)-ibuprofen
70. Artril 300
71. 4-isobutyl-alpha-methylphenylacetic Acid
72. Midol 200
73. Duralbuprofen
74. Butacortelone
75. Amibufen
76. Caldolor
77. Dolgirid
78. Ibuprofeno
79. Ibuprofenum
80. Lebrufen
81. Seclodin
82. Amelior
83. Femadon
84. Ibutid
85. Inoven
86. Alpha-(4-isobutylphenyl)propionic Acid
87. Brufen Retard
88. Ibu-attritin
89. Dolo-dolgit
90. Aches-n-pain
91. Ip-82
92. (+-)-p-isobutylhydratropic Acid
93. Pedea
94. Rd 13621
95. Hydratropic Acid, P-isobutyl-
96. Ib-100
97. (4-isobutylphenyl)-alpha-methylacetic Acid
98. 58560-75-1
99. U-18,573
100. P-isobutyl-2-phenylpropionic Acid
101. (+-)-2-(p-isobutylphenyl)propionic Acid
102. Alpha-(p-isobutylphenyl)propionic Acid
103. 2-(4-isobutylphenyl)propionic Acid
104. Alpha-p-isobutylphenylpropionic Acid
105. (+-)-alpha-methyl-4-(2-methylpropyl)benzeneacetic Acid
106. Benzeneacetic Acid, .alpha.-methyl-4-(2-methylpropyl)-
107. Chebi:5855
108. Alpha-methyl-4-(2-methylpropyl)benzeneacetic Acid
109. Benzeneacetic Acid, Alpha-methyl-4-(2-methylpropyl)-
110. M01ae01
111. (+/-)-p-isobutylhydratropic Acid
112. 2-(p-isobutylphenyl)propionic Acid
113. Novoprofen
114. Amersol
115. Dolocyl
116. Lidifen
117. Mfcd00010393
118. Paxofen
119. Rafen
120. Wk2xyi10qm
121. Ibu
122. Chembl521
123. Ifen
124. U-18573
125. 2-(4-isobutyl-phenyl)-propionic Acid
126. Nsc-256857
127. Alpha-methyl-4-(isobutyl)phenylacetic Acid
128. Mls000069733
129. Balkaprofen
130. Betaprofen
131. Daiprophen
132. Jenaprofen
133. Antagil
134. Antalfene
135. Antarene
136. Antiflam
137. Artofen
138. Bruflam
139. Bufigen
140. Bukrefen
141. Buracaps
142. Citalgan
143. Combiflam
144. Dansida
145. Dentigoa
146. Dignoflex
147. Dolmaral
148. Dolofen
149. Dolofin
150. Dolofort
151. Dologel
152. Dolomax
153. Doloren
154. Doltibil
155. Dorival
156. Duobrus
157. Dysdolen
158. Easifon
159. Esprenit
160. Exneural
161. Femafen
162. Femapirin
163. Femidol
164. Fenspan
165. Fibraflex
166. Gelufene
167. Gynofug
168. Ibubest
169. Ibubeta
170. Ibucasen
171. Ibudolor
172. Ibuflamar
173. Ibugesic
174. Ibuhexal
175. Ibulagic
176. Ibuleve
177. Ibulgan
178. Ibumerck
179. Ibupirac
180. Junifen
181. Kratalgin
182. Librofem
183. Malafene
184. Manypren
185. Mensoton
186. Neobrufen
187. Nerofen
188. Noalgil
189. Nobafon
190. Noritis
191. Novadol
192. Novogent
193. Nuprilan
194. Opturem
195. Oralfene
196. Ostarin
197. Paduden
198. Perofen
199. R.d. 13621
200. Ranofen
201. Relcofen
202. Rhinadvil
203. Sadefen
204. Salivia
205. Sednafen
206. Seklodin
207. Seskafen
208. Siyafen
209. Solpaflex
210. Sugafen
211. Suprafen
212. Syntofene
213. Tatanal
214. Unipron
215. Alaxan
216. Anafen
217. Artril
218. Brofen
219. Bufeno
220. Bupron
221. Dolibu
222. Dolven
223. Duafen
224. Emflam
225. Eputex
226. Faspic
227. Fenbid
228. Fendol
229. Grefen
230. Ibudol
231. Ibufug
232. Ibugel
233. Ibugen
234. Ibular
235. Ibulav
236. Ibumed
237. Ibusal
238. Inflam
239. Isodol
240. Lopane
241. Melfen
242. Moment
243. Narfen
244. Ozonol
245. Provon
246. Stelar
247. Tempil
248. Bloom
249. Cesra
250. Cunil
251. Dalsy
252. Ergix
253. Gofen
254. Ipren
255. Irfen
256. Kesan
257. Rofen
258. Rufin
259. Rupan
260. Tofen
261. Tonal
262. Upfen
263. Zafen
264. Zofen
265. Drin
266. Ibol
267. Pediatric Advil
268. Apo-ibuprofen
269. Neo-helvagit
270. Deep Relief
271. Ibu-slow
272. Novo-profen
273. Neo-mindol
274. Dolofen-f
275. Donjust B
276. Nagifen-d
277. Dura-ibu
278. Children's Advil
279. Motrin Ib
280. Togal N
281. Children's Motrin
282. Nsc256857
283. Ibu-tab 200
284. .alpha.-(p-isobutylphenyl)propionic Acid
285. Children's Ibuprofen
286. Ncgc00015529-09
287. Dularbuprofen
288. .alpha.-(4-isobutylphenyl)propionic Acid
289. Acide (isobutyl-4 Phenyl)-2 Propionique
290. Ibuprofene
291. Ibuprophen
292. Smr000058184
293. Tabalon 400
294. Junior Strength Advil
295. (+/-)-2-(p-isobutylphenyl)propionic Acid
296. 4-isobutyl-.alpha.-methylphenylacetic Acid
297. Brufen 400
298. Emflam-200
299. Kontagripp Mono
300. Dolo Puren
301. Dsstox_cid_732
302. Junior Strength Motrin
303. Novo Dioxadol
304. Schmerz-dolgit
305. Act-3
306. Ak+c2278tren
307. Hemagene Tailleur
308. Advil Liqui-gels
309. Adex 200
310. Junior Strength Ibuprofen
311. Dsstox_rid_75759
312. Dsstox_gsid_20732
313. Advil, Children's
314. Am-fam 400
315. Codral Period Pain
316. Children's Elixsure
317. (+-)-ibuprophen
318. .alpha.-methyl-4-(2-methylpropyl)benzeneacetic Acid
319. Midol Ib Cramp Relief
320. Alivium
321. Genpril
322. Advil Migraine
323. Bayer Select Pain Relief
324. Ibuprofene [inn-french]
325. Ibuprofenum [inn-latin]
326. Perrigo Ibuprofen
327. Racemic Ibuprofen
328. Ibuprofeno [inn-spanish]
329. Motrin Migraine Pain
330. Motrin Ib Gelcaps
331. Ucb 79171
332. Alpha-(4-isobutylphenyl)propionate
333. Cas-15687-27-1
334. Motrin (tn)
335. Ccris 3223
336. Advil (tn)
337. Children's Advil-flavored
338. Hsdb 3099
339. Children's Elixsure Ib
340. Sr-01000000214
341. Einecs 239-784-6
342. Unii-wk2xyi10qm
343. Advil Migraine Liqui-gels
344. U 18573
345. Nsc 256857
346. Brn 2049713
347. Ibupril
348. Ibuprox
349. Aktren
350. Ibux
351. Rac Ibuprofen
352. (+/-)-2-(4-isobutylphenyl)propanoic Acid
353. Cdt-ibuprofen
354. Mfcd00069289
355. Nurofen Meltlets
356. (y)-ibuprofen
357. Ibuprofen,(s)
358. Midol Liquid Gels
359. (+) Ibuprofen
360. Ibuprofen (advil)
361. Acide (isobutyl-4-phenyl)-2 Propionique [french]
362. (?)-ibuprofen
363. Ibuprofen [usan]
364. (s)-ibuprofen-d3
365. P-isobutylhydratropate
366. Ibuprofen [usan:usp:inn:ban:jan]
367. Combunox (salt/mix)
368. (a+/-)-ibuprofen
369. Spectrum_000849
370. Acide (isobutyl-4-phenyl)-2 Propionique
371. Ibuprofen [inn]
372. Ibuprofen [jan]
373. Opera_id_554
374. Ibuprofen [mi]
375. Ibuprofen [hsdb]
376. Ibuprofen [inci]
377. Spectrum2_000129
378. Spectrum3_000465
379. Spectrum4_000015
380. Spectrum5_000862
381. Ibuprofen [vandf]
382. Wln: Qvy&r Diy
383. Cbmicro_005634
384. Epitope Id:139973
385. Ibuprofen [mart.]
386. P-isobutyl-hydratropic Acid
387. Ec 239-784-6
388. I 4883
389. Cambridge Id 5152402
390. Ibuprofen [usp-rs]
391. Ibuprofen [who-dd]
392. Ibuprofen [who-ip]
393. Schembl3001
394. Lopac0_000691
395. Bspbio_002170
396. Ibuprofen - Adooq Bioscience
397. Ibuprofen [ema Epar]
398. Ibuprofen-[u-ring-13c6]
399. Kbiogr_000389
400. Kbioss_001329
401. Mls001146965
402. P-isobutyl-2-phenylpropionate
403. Bidd:gt0050
404. Divk1c_000887
405. Spectrum1500347
406. Ibuprofen, >=98% (gc)
407. Spbio_000178
408. Ibuprofen (jp17/usp/inn)
409. Alpha-p-isobutylphenylpropionate
410. Gtpl2713
411. Motrin Ib Gelcaps (salt/mix)
412. Ibuprofen [orange Book]
413. 2-p-isobutylphenylpropionic Acid
414. Dtxsid5020732
415. Ibuprofen [ep Monograph]
416. Ibuprofen [usp Impurity]
417. Hefnnwsxxwatrw-uhfffaoysa-
418. Hms502m09
419. Kbio1_000887
420. Kbio2_001329
421. Kbio2_003897
422. Kbio2_006465
423. Kbio3_001390
424. Advil Cold & Sinus (salt/mix)
425. Ibuprofen [usp Monograph]
426. Ibuprofen 1.0 Mg/ml In Methanol
427. Ninds_000887
428. Sine-aid Ib Caplets (salt/mix)
429. Benzeneacetic Acid, Alpha-methyl-4-(2-methylpropyl), (+-)-
430. Hms1920f15
431. Hms2089p05
432. Hms2091n03
433. Hms2230n04
434. Hms3259g05
435. Hms3262k03
436. Hms3372m09
437. Hms3649m11
438. Hms3651a15
439. Hms3884i04
440. Pharmakon1600-01500347
441. Combunox Component Ibuprofen
442. Ibuprofenum [who-ip Latin]
443. Act03248
444. Albb-025647
445. Bcp10423
446. Bcp20325
447. Bcp25225
448. Reprexain Component Ibuprofen
449. Zag-1701
450. ( Inverted Question Mark)-ibuprofen
451. (.+-.)-p-isobutylhydratropic Acid
452. Children's Elixsure Ib (salt/mix)
453. Tox21_110170
454. Tox21_201384
455. Tox21_302829
456. Tox21_500691
457. 1189866-35-0 (unlabeled)
458. 2-(4'-isobutylphenyl)propionic Acid
459. 2-(4-isobutylphenyl) Propanoic Acid
460. 2-(4-isobutylphenyl) Propionic Acid
461. 2-(4-isobutylphenyl)-propionic Acid
462. Bbl010660
463. Bdbm50009859
464. Ccg-38947
465. Nsc757073
466. S1638
467. Stk177358
468. Vicoprofen Component Ibuprofen
469. (.+/-.)-p-isobutylhydratropic Acid
470. 2-(4-isobutylphenyl)-propionoic Acid
471. Ibuprofen 100 Microg/ml In Methanol
472. 2-(4'-isobutylphenyl)-propionic Acid
473. Akos003237488
474. Akos016340658
475. Ibuprofen Component Of Combunox
476. Propanoic Acid, 2-(4-isobutylphenyl)
477. Tox21_110170_1
478. ( Inverted Exclamation Marka)-ibuprofe
479. Cs-1931
480. Db01050
481. Ibuprofen Component Of Reprexain
482. Ks-5029
483. Lp00691
484. Nc00458
485. Nsc-757073
486. Sb19113
487. Sdccgsbi-0050669.p005
488. Sine-aid Ib Component Ibuprofen
489. St-1482
490. ( Inverted Exclamation Marka)-ibuprofen
491. Alpha-(4-isobutylphenyl)-propionic Acid
492. Alpha-(p-isobutylphenyl)-propionic Acid
493. Ibuprofen Component Of Vicoprofen
494. Idi1_000887
495. 2-(4-isobutylphenyl)propionic Acid-d3;
496. Ncgc00015529-04
497. Ncgc00015529-05
498. Ncgc00015529-06
499. Ncgc00015529-07
500. Ncgc00015529-08
501. Ncgc00015529-10
502. Ncgc00015529-12
503. Ncgc00015529-13
504. Ncgc00015529-14
505. Ncgc00015529-15
506. Ncgc00015529-18
507. Ncgc00015529-32
508. Ncgc00089819-02
509. Ncgc00089819-03
510. Ncgc00089819-04
511. Ncgc00089819-05
512. Ncgc00089819-06
513. Ncgc00089819-07
514. Ncgc00256416-01
515. Ncgc00258935-01
516. Ncgc00261376-01
517. Ac-11312
518. Bi166241
519. Hy-78131
520. Ibuprofen Component Of Sine-aid Ib
521. Nci60_002065
522. Sy046826
523. Ibuprofen, Vetec(tm) Reagent Grade, 97%
524. Sbi-0050669.p004
525. .alpha.-2-(p-isobutylphenyl)propionic Acid
526. Db-043333
527. Db-071312
528. Ibuprofen, Meets Usp Testing Specifications
529. (.+-.)-2-(p-isobutylphenyl)propionic Acid
530. 4-isobutylphenyl)-.alpha.-methylacetic Acid
531. Am20060782
532. Bb 0258487
533. Eu-0100691
534. Ft-0601629
535. Ft-0642971
536. Ft-0655194
537. Ft-0670257
538. Ft-0670258
539. I0415
540. Sw203738-2
541. (.+/-.)-2-(p-isobutylphenyl)propionic Acid
542. Advil Allergy Sinus Component Ibuprofen
543. Ibuprofen, Vetranal(tm), Analytical Standard
544. (r/s)-alpha-methyl-4-isobutylphenylacetic Acid
545. C01588
546. D00126
547. Ab00052020-16
548. Ab00052020-17
549. Ab00052020_18
550. Ab00052020_19
551. Children's Advil Cold Component Ibuprofen
552. 687i271
553. A831926
554. Advil Congestion Relief Component Ibuprofen
555. Children's Motrin Cold Component Ibuprofen
556. Ibuprofen Component Of Advil Allergy Sinus
557. Q186969
558. Ibuprofen Component Of Children's Advil Cold
559. Ibuprofen Component Of Children's Motrin Cold
560. J-009349
561. Sr-01000000214-2
562. Sr-01000000214-4
563. Brd-a17655518-001-02-0
564. Brd-a17655518-001-12-9
565. Ibuprofen Component Of Advil Congestion Relief
566. Sr-01000000214-13
567. F2173-0233
568. Ibuprofen, British Pharmacopoeia (bp) Reference Standard
569. Z1695709473
570. (+/-)-alpha-methyl-4-(2-methylpropyl)benzeneacetic Acid
571. Ibuprofen, European Pharmacopoeia (ep) Reference Standard
572. Ibuprofen, United States Pharmacopeia (usp) Reference Standard
573. Benzeneacetic Acid, .alpha.-methyl-4-(2-methylpropyl), (.+/-.)-
574. Benzeneacetic Acid, .alpha.-methyl-4-(2-methylpropyl), (+/-)-
575. Dexibuprofen;(s)-ibuprofen; L 669455; L-669,455, Mk 233; Mk-233
576. Ibuprofen, Pharmaceutical Secondary Standard; Certified Reference Material
577. Ibuprofen For Peak Identification, European Pharmacopoeia (ep) Reference Standard
578. 2-(4-isobutylphenyl)-propionic Acid ; Alpha-methyl-4-(2-methylpropyl)benzeneacetic Acid ; P-isobutylhydratropic Acid
| Molecular Weight | 206.28 g/mol |
|---|---|
| Molecular Formula | C13H18O2 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 206.130679813 g/mol |
| Monoisotopic Mass | 206.130679813 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 203 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 44 | |
|---|---|
| Drug Name | Ibuprofen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet; Capsule; Suspension; Suspension/drops; Tablet, chewable |
| Route | Oral |
| Strength | 200mg; eq 200mg free acid and potassium salt; 100mg/5ml; 600mg; 800mg; 400mg; 300mg; 100mg; 40mg/ml; 50mg |
| Market Status | Over the Counter; Prescription |
| Company | Vintage Pharms; Amneal Pharms; Marksans Pharma; Merro Pharm; Dr Reddys La; Pld Acquisitions; Lnk; Banner Pharmacaps; Mcneil; Ohm; Par Pharm; Granules India; Perrigo R And D; Svads Holdings Sa; Amneal Pharms Ny; Shasun Usa; Actavis Mid Atlantic; Perrigo; C |
| 2 of 44 | |
|---|---|
| Drug Name | Ibuprohm |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Ohm Labs |
| 3 of 44 | |
|---|---|
| Drug Name | Ibu-tab |
| Drug Label | IBU tablets contain the active ingredient ibuprofen, which is (-2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77C and is very slightly soluble in water( |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg; 400mg |
| Market Status | Prescription |
| Company | Alra |
| 4 of 44 | |
|---|---|
| Drug Name | Ibu-tab 200 |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Alra |
| 5 of 44 | |
|---|---|
| Drug Name | Junior strength advil |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 100mg |
| Market Status | Over the Counter |
| Company | Pfizer |
| 6 of 44 | |
|---|---|
| Drug Name | Junior strength ibuprofen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg |
| Market Status | Over the Counter |
| Company | Perrigo |
| 7 of 44 | |
|---|---|
| Drug Name | Junior strength motrin |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 100mg |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
| 8 of 44 | |
|---|---|
| Drug Name | Motrin ib |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Mcneil |
| 9 of 44 | |
|---|---|
| Drug Name | Motrin migraine pain |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Mcneil |
| 10 of 44 | |
|---|---|
| Drug Name | Neoprofen |
| Active Ingredient | Ibuprofen lysine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | eq 20mg base/2ml (eq 10mg base/ml) |
| Market Status | Prescription |
| Company | Recordati Rare |
| 11 of 44 | |
|---|---|
| Drug Name | Pediatric advil |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension/drops |
| Route | Oral |
| Strength | 100mg/2.5ml |
| Market Status | Over the Counter |
| Company | Pfizer |
| 12 of 44 | |
|---|---|
| Drug Name | Profen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Contract Pharmacal |
| 13 of 44 | |
|---|---|
| Drug Name | Tab-profen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Perrigo |
| 14 of 44 | |
|---|---|
| Drug Name | Advil |
| PubMed Health | Ibuprofen |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Ibuprofen sodium; Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; eq 200mg base |
| Market Status | Over the Counter |
| Company | Pfizer Cons Hlthcare; Pfizer |
| 15 of 44 | |
|---|---|
| Drug Name | Advil liqui-gels |
| PubMed Health | Ibuprofen |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Caldolor contains the active ingredient ibuprofen, which is ()-2-(p-isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77C. It has a molecular weight of 206.28. It is very slightly soluble in water ( |
| Active Ingredient | Ibuprofen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg free acid and potassium salt |
| Market Status | Over the Counter |
| Company | Pfizer |
| 16 of 44 | |
|---|---|
| Drug Name | Advil migraine liqui-gels |
| PubMed Health | Ibuprofen |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Ibuprofen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg free acid and potassium salt |
| Market Status | Over the Counter |
| Company | Pfizer |
| 17 of 44 | |
|---|---|
| Drug Name | Caldolor |
| PubMed Health | Ibuprofen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Ibuprofen |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 800mg/8ml (100mg/ml) |
| Market Status | Prescription |
| Company | Cumberland Pharms |
| 18 of 44 | |
|---|---|
| Drug Name | Children's advil |
| Drug Label | IBU tablets contain the active ingredient ibuprofen, which is (-2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77C and is very slightly soluble in water( |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet, chewable; Suspension |
| Route | Oral |
| Strength | 50mg; 100mg/5ml |
| Market Status | Over the Counter |
| Company | Pfizer |
| 19 of 44 | |
|---|---|
| Drug Name | Children's advil-flavored |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 100mg/5ml |
| Market Status | Over the Counter |
| Company | Pfizer |
| 20 of 44 | |
|---|---|
| Drug Name | Children's elixsure |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 100mg/5ml |
| Market Status | Over the Counter |
| Company | Moberg Pharma North |
| 21 of 44 | |
|---|---|
| Drug Name | Children's ibuprofen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 100mg/5ml |
| Market Status | Over the Counter |
| Company | Perrigo |
| 22 of 44 | |
|---|---|
| Drug Name | Children's motrin |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension/drops; Tablet, chewable; Suspension |
| Route | Oral |
| Strength | 40mg/ml; 50mg; 100mg/5ml |
| Market Status | Over the Counter |
| Company | Mcneil Cons; Mcneil |
| 23 of 44 | |
|---|---|
| Drug Name | Ibuprofen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet; Capsule; Suspension; Suspension/drops; Tablet, chewable |
| Route | Oral |
| Strength | 200mg; eq 200mg free acid and potassium salt; 100mg/5ml; 600mg; 800mg; 400mg; 300mg; 100mg; 40mg/ml; 50mg |
| Market Status | Over the Counter; Prescription |
| Company | Vintage Pharms; Amneal Pharms; Marksans Pharma; Merro Pharm; Dr Reddys La; Pld Acquisitions; Lnk; Banner Pharmacaps; Mcneil; Ohm; Par Pharm; Granules India; Perrigo R And D; Svads Holdings Sa; Amneal Pharms Ny; Shasun Usa; Actavis Mid Atlantic; Perrigo; C |
| 24 of 44 | |
|---|---|
| Drug Name | Ibuprohm |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Ohm Labs |
| 25 of 44 | |
|---|---|
| Drug Name | Ibu-tab |
| Drug Label | IBU tablets contain the active ingredient ibuprofen, which is (-2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77C and is very slightly soluble in water( |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg; 400mg |
| Market Status | Prescription |
| Company | Alra |
| 26 of 44 | |
|---|---|
| Drug Name | Ibu-tab 200 |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Alra |
| 27 of 44 | |
|---|---|
| Drug Name | Junior strength advil |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 100mg |
| Market Status | Over the Counter |
| Company | Pfizer |
| 28 of 44 | |
|---|---|
| Drug Name | Junior strength ibuprofen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg |
| Market Status | Over the Counter |
| Company | Perrigo |
| 29 of 44 | |
|---|---|
| Drug Name | Junior strength motrin |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet; Tablet, chewable |
| Route | Oral |
| Strength | 100mg |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
| 30 of 44 | |
|---|---|
| Drug Name | Motrin ib |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Mcneil |
| 31 of 44 | |
|---|---|
| Drug Name | Motrin migraine pain |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Mcneil |
| 32 of 44 | |
|---|---|
| Drug Name | Neoprofen |
| Active Ingredient | Ibuprofen lysine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | eq 20mg base/2ml (eq 10mg base/ml) |
| Market Status | Prescription |
| Company | Recordati Rare |
| 33 of 44 | |
|---|---|
| Drug Name | Pediatric advil |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension/drops |
| Route | Oral |
| Strength | 100mg/2.5ml |
| Market Status | Over the Counter |
| Company | Pfizer |
| 34 of 44 | |
|---|---|
| Drug Name | Profen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Contract Pharmacal |
| 35 of 44 | |
|---|---|
| Drug Name | Tab-profen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg |
| Market Status | Over the Counter |
| Company | Perrigo |
| 36 of 44 | |
|---|---|
| Drug Name | Advil |
| PubMed Health | Ibuprofen |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Ibuprofen sodium; Ibuprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; eq 200mg base |
| Market Status | Over the Counter |
| Company | Pfizer Cons Hlthcare; Pfizer |
| 37 of 44 | |
|---|---|
| Drug Name | Advil liqui-gels |
| PubMed Health | Ibuprofen |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Caldolor contains the active ingredient ibuprofen, which is ()-2-(p-isobutylphenyl) propionic acid. Ibuprofen is a white powder with a melting point of 74-77C. It has a molecular weight of 206.28. It is very slightly soluble in water ( |
| Active Ingredient | Ibuprofen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg free acid and potassium salt |
| Market Status | Over the Counter |
| Company | Pfizer |
| 38 of 44 | |
|---|---|
| Drug Name | Advil migraine liqui-gels |
| PubMed Health | Ibuprofen |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Ibuprofen |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 200mg free acid and potassium salt |
| Market Status | Over the Counter |
| Company | Pfizer |
| 39 of 44 | |
|---|---|
| Drug Name | Caldolor |
| PubMed Health | Ibuprofen (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Active Ingredient | Ibuprofen |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 800mg/8ml (100mg/ml) |
| Market Status | Prescription |
| Company | Cumberland Pharms |
| 40 of 44 | |
|---|---|
| Drug Name | Children's advil |
| Drug Label | IBU tablets contain the active ingredient ibuprofen, which is (-2 - (p - isobutylphenyl) propionic acid. Ibuprofen is a white powde rwith a melting point of 74-77C and is very slightly soluble in water( |
| Active Ingredient | Ibuprofen |
| Dosage Form | Tablet, chewable; Suspension |
| Route | Oral |
| Strength | 50mg; 100mg/5ml |
| Market Status | Over the Counter |
| Company | Pfizer |
| 41 of 44 | |
|---|---|
| Drug Name | Children's advil-flavored |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 100mg/5ml |
| Market Status | Over the Counter |
| Company | Pfizer |
| 42 of 44 | |
|---|---|
| Drug Name | Children's elixsure |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 100mg/5ml |
| Market Status | Over the Counter |
| Company | Moberg Pharma North |
| 43 of 44 | |
|---|---|
| Drug Name | Children's ibuprofen |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension |
| Route | Oral |
| Strength | 100mg/5ml |
| Market Status | Over the Counter |
| Company | Perrigo |
| 44 of 44 | |
|---|---|
| Drug Name | Children's motrin |
| Active Ingredient | Ibuprofen |
| Dosage Form | Suspension/drops; Tablet, chewable; Suspension |
| Route | Oral |
| Strength | 40mg/ml; 50mg; 100mg/5ml |
| Market Status | Over the Counter |
| Company | Mcneil Cons; Mcneil |
Analgesics, Non-Narcotic; Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ibuprofen ... /is/ indicated for reduction of fever. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 415
Ibuprofen ... /is/ used for relief of the pain and inflammation of acute gouty arthritis and acute calcium pyrophosphate deposition disease (pseudogout; chondrocalcinosis articularis; synovitis, crystal-induced). Only immediate-release dosage forms are recommended for relief of acute attacks because of their more rapid onset of action relative to delayed-release or extended-release dosage forms. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 414
Ibuprofen ... /is/ indicated for relief of mild to moderate pain, especially when anti-inflammatory actions may also be desired, e.g., following dental, obstetric, or orthopedic surgery, and for relief of musculoskeletal pain due to soft tissue athletic injuries (strains or sprains). Only immediate-release dosage forms are recommended for relief of acute pain because of their more rapid onset of actin relative to delayed-release or extended-release dosage forms. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 414
For more Therapeutic Uses (Complete) data for IBUPROFEN (28 total), please visit the HSDB record page.
Ibuprofen should be used with caution in patients with peptic ulcer disease, GI perforation or bleeding, bleeding abnormalities (especially in patients who may be adversely affected by prolongation of bleeding time), impaired renal function, hypertension, or compromised cardiac function.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1971
CAUTION IN THE USE OF IBUPROFEN IN SYSTEMIC LUPUS ERYTHEMATOSUS IS ADVISED, PARTICULARLY IF THERE IS HISTORY OF SALICYLATE INTOLERANCE.
PMID:439358 FINCH WR, STROTTMAN MP; JAMA 241 (JUN 15): 2616 (1979)
Ibuprofen is not recommended for use by pregnant women, or by those who are breast-feeding their infants.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 712
IBUPROFEN ELEVATES BILIRUBIN, ALKALINE PHOSPHATASE, ASPARTATE AMINOTRANSFERASE (SGOT), AND ALANINE AMINOTRANSFERASE (SGPT) ABOVE THE NORMAL RANGE, AND CAUSES ISOLATED CASES OF JAUNDICE. /FROM TABLE/
Hansten, P.D. Drug Interactions. 4th ed. Philadelphia: Lea and Febiger, 1979., p. 504
For more Drug Warnings (Complete) data for IBUPROFEN (14 total), please visit the HSDB record page.
Ibuprofen is the most commonly used and prescribed NSAID. It is very common over the counter medication widely used as an analgesic, anti-inflammatory and antipyretic. The use of ibuprofen and its enantiomer [DB09213] in a racemic mix is common for the management of mild to moderate pain related to dysmenorrhea, headache, migraine, postoperative dental pain, spondylitis, osteoarthritis, rheumatoid arthritis, and soft tissue disorder. Due to its activity against prostaglandin and thromboxane synthesis, ibuprofen has been attributed to alteration of platelet function and prolongation of gestation and labor. As ibuprofen is a widely used medication, the main therapeutic indications are: * Patent Ductus Arteriosus - it is a neonatal condition wherein the ductus arteriosus (blood vessel that connects the main pulmonary artery to the proximal descending aorta) fails to close after birth causing severe risk of heart failure. The prostaglandin inhibition of ibuprofen has been studied for the treatment of this condition as it is known that prostaglandin E2 is responsible for keeping the ductus arteriosus open. * Rheumatoid- and osteo-arthritis - ibuprofen is very commonly used in the symptomatic treatment of inflammatory, musculoskeletal and rheumatic disorders. * Cystic fibrosis - the use of high dosages of ibuprofen has been proven to decrease inflammation and decreasing polymorphonuclear cell influx in the lungs. * Orthostatic hypotension - ibuprofen can induce sodium retention and antagonize the effect of diuretics which has been reported to be beneficial for patients with severe orthostatic hypotension. * Dental pain - ibuprofen is used to manage acute and chronic orofacial pain. * Minor pain - ibuprofen is widely used to reduce minor aches and pains as well as to reduce fever and manage dysmenorrhea. It is very commonly used for the relief of acute indications such as fever and tension headaches. * Investigational uses - efforts have been put into developing ibuprofen for the prophylaxis of Alzheimer's disease, Parkinson disease, and breast cancer.
FDA Label
Treatment of a haemodynamically significant patent ductus arteriosus in preterm newborn infants less than 34 weeks of gestational age.
Treatment of pain
Treatment of febrile disorders, Treatment of pain
Treatment of febrile disorders, Treatment of pain
Ibuprofen has multiple actions in different inflammatory pathways involved in acute and chronic inflammation. The main effects reported in ibuprofen are related to the control of pain, fever and acute inflammation by the inhibition of the synthesis of prostanoids by COX-1 and COX-2. Pain relief is attributed to peripheral affected regions and central nervous system effects in the pain transmission mediated by the dorsal horn and higher spinothalamic tract. Some reports have tried to link the pain regulation with a possible enhancement on the synthesis of endogenous cannabinoids and action on the NMDA receptors. The effect on pain has been shown to be related to the cortically evoked potentials. The antipyretic effect is reported to be linked to the effect on the prostanoid synthesis due to the fact that the prostanoids are the main signaling mediator of pyresis in the hypothalamic-preoptic region. The use of ibuprofen in dental procedures is attributed to the local inhibition of prostanoid production as well as to anti-oedemic activity and an increase of plasma beta-endorphins. Some reports have suggested a rapid local reduction of the expression of COX-2 in dental pulp derived by the administration of ibuprofen. The administration of ibuprofen in patients with rheumatic diseases has shown to control joint symptoms. Ibuprofen is largely used in OTC products such as an agent for the management of dysmenorrhea which has been proven to reduce the amount of menstrual prostanoids and to produce a reduction in the uterine hypercontractility. As well, it has been reported to reduce significantly the fever and the pain caused by migraines. This effect is thought to be related to the effect on platelet activation and thromboxane A2 production which produces local vascular effects in the affected regions. This effect is viable as ibuprofen can enter in the central nervous system. In the investigational uses of ibuprofen, it has been reported to reduce neurodegeneration when given in low doses over a long time. On the other hand, its use in Parkinson disease is related to the importance of inflammation and oxidative stress in the pathology of this condition. The use of ibuprofen for breast cancer is related to a study that shows a decrease of 50% in the rate of breast cancer.
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
C01EB16
M01AE01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01E - Other cardiac preparations
C01EB - Other cardiac preparations
C01EB16 - Ibuprofen
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CC - Antiinflammatory products for vaginal administration
G02CC01 - Ibuprofen
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE01 - Ibuprofen
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA13 - Ibuprofen
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AX - Other throat preparations
R02AX02 - Ibuprofen
Absorption
It is very well absorbed orally and the peak serum concentration can be attained in 1 to 2 hours after extravascular administration. When ibuprofen is administered immediately after a meal there is a slight reduction in the absorption rate but there is no change in the extent of the absorption. When orally administered, the absorption of ibuprofen in adults is very rapidly done in the upper GI tract. The average Cmax, Tmax and AUC ranges around 20 mcg/ml, 2 h and 70 mcg.h/ml. These parameters can vary depending on the enantiomer form, route, and dose of administration.
Route of Elimination
Ibuprofen is rapidly metabolized and eliminated in the urine thus, this via accounts for more than 90% of the administered dose. It is completely eliminated in 24 hours after the last dose and almost all the administered dose goes through metabolism, representing about 99% of the eliminated dose. The biliary excretion of unchanged drug and active phase II metabolites represents 1% of the administered dose. In summary, ibuprofen is excreted as metabolites or their conjugates. The elimination of ibuprofen is not impaired by old age or the presence of renal impairment.
Volume of Distribution
The apparent volume of distribution of ibuprofen is of 0.1 L/kg.
Clearance
The clearance rate ranges between 3-13 L/h depending on the route of administration, enantiomer type and dosage.
Ibuprofen is rapidly absorbed after oral admin, & peak concns in plasma are observed after 15-30 min. The half-life in plasma is about 2 hr. Ibuprofen is extensively (99%) bound to plasma proteins, but the drug occupies only a fraction of the total drug-binding sites at usual concns. Ibuprofen passes slowly into the synovial spaces & may remain there in higher concn as the concns in plasma decline. In experimental animals, ibuprofen & its metabolites pass easily across the placenta. The excretion of ibuprofen is rapid & complete. More than 90% of an ingested dose is excreted in the urine as metabolites or their conjugates.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 712
ENTERO-HEPATIC CIRCULATION OF (14)C-IBUPROFEN & ITS METABOLITES MAY HAVE OCCURRED IN DOGS RECEIVING REPEATED ORAL DOSES ... SINCE LEVELS IN BILE ... WERE 40-FOLD THOSE IN PLASMA.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 47
AFTER ORAL DOSES OF 400 MG IBUPROFEN, SERIAL BLOOD SAMPLES WERE TAKEN (5 MALE VOLUNTEERS, 4 ARTHRITIC PATIENT). EVIDENCE SHOWED 2 COMPARTMENT MODEL: NO EVIDENCE SHOWN OF DRUG ACCUM IN PERIPHERAL COMPARTMENT.
COLLIER PS ET AL; BR J CLIN PHARMACOL 5(JUNE) 528 (1978)
The enantiomeric composition of ibuprofen in plasma was investigated following oral administration of 200 mg of the racemic drug in a conventional tablet or 300 mg in a novel controlled release pellet formulation to 4 healthy volunteers, aged 24 to 37 yr, in a randomized, crossover study. The plasma concentration time profiles suggest that drug release from the controlled release preparation was suitably modified and that the fluctuation between the peaks and troughs observed following a conventional tablet formulation were reduced. The plasma concentrations of (+)-ibuprofen (S-ibuprofen) were greater than those of (-)-ibuprofen (R-ibuprofen) following either formulation, and the enantiomeric plasma ratio (S/R) was reduced, both in magnitude and variability, following the controlled release preparation. The proportion of the total area under the plasma concentration time curves, due to (S)-ibuprofen, were slightly reduced following the controlled release formulation compared to the tablet formulation. The importance of a consideration of stereochemistry in bioequivalence studies of chiral drugs is discussed.
Avgerinos A et al; Int J Pharm 68 (Feb 1): 97-103 (1991)
For more Absorption, Distribution and Excretion (Complete) data for IBUPROFEN (11 total), please visit the HSDB record page.
Ibuprofen is rapidly metabolized and biotransformed in the liver to the formation of major metabolites which are the hydroxylated and carboxylated derivatives. As soon as it is absorbed, the R-enantiomer undergoes extensive enantiomeric conversion (53-65%) to the more active S-enantiomer _in vivo_ by the activity of alpha-methylacyl-CoA racemase. Ibuprofen metabolism can be divided in phase I which is represented by the hydroxylation of the isobutyl chains for the formation of 2 or 3-hydroxy derivatives followed by oxidation to 2-carboxy-ibuprofen and p-carboxy-2-propionate. These oxidative reactions are performed by the activity of the cytochrome P450 isoforms CYP 2C9, CYP 2C19 and CYP 2C8. Therefore, these enzymes participate in the oxidation of the alkyl side chain to hydroxyl and carboxyl derivatives. From this enzymes, the major catalyst in the formation of oxidative metabolites is the isoform CYP 2C9. The metabolic phase I is followed by a phase II in which the oxidative metabolites may be conjugated to glucuronide prior to excretion. This activity forms phenolic and acyl glucuronides.
TWO MAJOR METABOLIC PATHWAYS IN MAN & IN ANIMALS PROCEED BY OXIDATIVE ATTACK OF ISOBUTYL SIDE CHAIN; THEY ARE HYDROXYLATION OF THE TERTIARY CARBON TO YIELD A STABLE TERTIARY ALCOHOL, & OXIDATION OF 1 OF THE 2 GEMINAL METHYL GROUPS TO YIELD THE ACID.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 22
IBUPROFEN GIVES 2-(4-(2-CARBOXYPROPYL)PHENYL)PROPIONIC ACID & 2-(4-(2-HYDROXY-2-METHYLPROPYL)PHENYL)PROPIONIC ACID IN MAN. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. I-14
The pharmacokinetics of oral ibuprofen following a dose of 0.8 g given 3 times a day for 14 days were studied in 7 functionally anephric patients (aged 34-66 yr) undergoing hemodialysis. No accumulation of ibuprofen plasma concns & an absence of intact ibuprofen in dialysate indicated clearance through metabolic pathways. The metabolites did accumulate significantly with mean plasma levels of 249 mcg/ml for the carboxy derivatives & 57 mcg/ml for the hydroxy derivatives of ibuprofen. However, both were detected in the dialysate. Dialysis clearance calculated by arterial & venous difference was found to agree with actual recovery in dialysate for both metabolites. Side effects were not observed in any subject.
PMID:3958223 Antal EJ et al; J Clin Pharmacol 26 (Mar): 184-90 (1986)
The serum half-life of ibuprofen is 1.2-2 hours. In patients with a compromised liver function, the half-life can be prolonged to 3.1-3.4 hours.
... After oral admin ... the half-life in plasma is about 2 hr.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 712
The exact mechanism of action of ibuprofen is unknown. However, ibuprofen is considered an NSAID and thus it is a non-selective inhibitor of cyclooxygenase, which is an enzyme involved in prostaglandin (mediators of pain and fever) and thromboxane (stimulators of blood clotting) synthesis via the arachidonic acid pathway. Ibuprofen is a non-selective COX inhibitor and hence, it inhibits the activity of both COX-1 and COX-2. The inhibition of COX-2 activity decreases the synthesis of prostaglandins involved in mediating inflammation, pain, fever, and swelling while the inhibition of COX-1 is thought to cause some of the side effects of ibuprofen including GI ulceration.
IBUPROFEN AT 25 MG/KG IV INCREASED THE PRIMARY AND TOTAL HEMOSTATIC PLUG FORMATION TIME IN RABBIT EAR CHAMBERS WITH LASER-INDUCED INJURY. THE SAME DOSE INCREASED THE NUMBER OF CUMULATIVE EMBOLI OVER A 10 MINUTE PERIOD AFTER A LASER INJURY TO ARTERIOLES. IN DOGS, DOSES OF 10, 25, AND 50 MG/KG DID NOT ENHANCE THE RELEASE OF (125)I-LABELED FIBRIN DEGRADATION PRODUCTS FROM THE THROMBI AFTER INCUBATION IN PLASMIN, BUT THE LARGEST DOSE SIGNIFICANTLY DECREASED THE THROMBUS WEIGHT 90 AND 180 MINUTES AFTER DRUG ADMINISTRATION. THUS, IBUPROFEN HAD AN INHIBITORY EFFECT ON PLATELET FUNCTION IN VIVO AND IN LARGE DOSES DIMINISHED THE THROMBUS WEIGHT.
ESQUIVEL CO ET AL; THROMB HAEMOSTASIS 48 (1): 87 (1982)
L-Arginine (L-arg) exhibits multiple biological properties and plays an important role in the regulation of different functions in pathological conditions. Many of these effects could be achieved on this amino acid serving as a substrate for the enzyme nitric oxide synthase (NOS). At the gastrointestinal level, recent reports revealed its protective activities involving a hyperemic response increasing the gastric blood flow. The aim of this study was to characterize the relationship between NOS activity/expression and prostaglandin changes (PGs) in rats gastric mucosa, with L-arg associated resistance to the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen (IBP). The protective effect of oral L-arg (100 mg/kg body wt), administerred together with IBP (100 mg/kg body wt, per os), was evident enough 90 min after drug administration, although a significant protection persisted for more than 6 hr. Pretreatment with N(G)-nitro-L-arginine (L-NNA) (40 mg/kg body wt, intraperitoneally), a competitive inhibitor of constitutive NOS, partly altered the protection afforded by the amino acid. In contrast, no changes could be observed after inducible NOS inhibition [aminoguanidine (AG) 50 mg/Kg body wt, intraperitoneally). L-arg, plus IBP, produced a significant increase of the cyclic GMP (cGMP) response in tissue samples from rat stomach, 90 min and 6 h after drug administration. iNOS activity and mRNA expression were higher in IBP-treated rats, and no differences were observed in inducible responses in the L-arg plus IBP group. No variations in the cNOS activity and expression were found among the different groups of animals assayed. The measurement of mucosal PGE2 content confirmed that biosynthesis of the eicosanoid is maintained by L-arg for over 90 min after IBP, while a total inhibition was observed 6 hr later. The mechanisms of the L-arg protective effect on the damaged induced by IBP could be explained by the different period after drug administration. The early phase is mediated by cyclooxygenase/prostaglandins pathway (COX/PGs) although NO liberated by cNOS and the guanylate cyclase/cGMP pathway could be also relevant. The later phase implicates inhibition of the iNOS/NO response.
PMID:11837731 Jimenez D et al; Dig Dis Sci 47 (1): 44-53 (2002)
We previously showed the non-steroidal anti-inflammatory drug (NSAID) ibuprofen suppresses inflammation and amyloid in the APPsw (Tg2576) Tg2576 transgenic mouse. The mechanism for these effects and the impact on behavior are unknown. We now show ibuprofen's effects were not mediated by alterations in amyloid precursor protein (APP) expression or oxidative damage (carbonyls). Six months ibuprofen treatment in Tg+ females caused a decrease in open field behavior (p < 0.05), restoring values similar to Tg- mice. Reduced caspase activation per plaque provided further evidence for a neuroprotective action of ibuprofen.The impact of a shorter 3 month duration ibuprofen trial, beginning at a later age (from 14 to 17 months), was also investigated. Repeated measures ANOVA of Abeta levels (soluble and insoluble) demonstrated a significant ibuprofen treatment effect (p < 0.05). Post-hoc analysis showed that ibuprofen-dependent reductions of both soluble Abeta and Abeta42 were most marked in entorhinal cortex (p < 0.05). Although interleukin-1beta and insoluble Abeta were more effectively reduced with longer treatment, the magnitude of the effect on soluble Abeta was not dependent on treatment duration.
PMID:11755007 Lim GP et al; Neurobiol Aging 22 (6): 983-91 (2001)
Trying to decrease the production of Amyloid beta (Abeta) has been envisaged as a promising approach to prevent neurodegeneration in Alzheimer's disease (AD). A chronic inflammatory reaction with activated microglia cells and astrocytes is a constant feature of AD. The participation of the immune system in the disease process is further documented in several retrospective clinical studies showing an inverse relationship between the prevalence of AD and nonsteroidal anti-inflammatory drug (NSAID) therapy. Previously, we demonstrated that the combination of the proinflammatory cytokines TNFalpha with IFNgamma induces the production of Abeta-42 and Abeta-40 in human neuronal cells. In the present study, the neuronal cell line Sk-n-sh was incubated for 12 h with the cyclooxygenase inhibitor ibuprofen and subsequently stimulated with the cytokines TNFalpha and IFNgamma. Ibuprofen treatment decreased the secretion of total Abeta in the conditioned media of cytokine stimulated cells by 50% and prevented the accumulation of Abeta-42 and Abeta-40 in detergent soluble cell extracts. Viability of neuronal cells measured by detection of apoptosis was neither influenced by ibuprofen nor by cytokine treatment. The reduction in the production of Abeta by ibuprofen was presumably due to a decreased production of betaAPP, which in contrast to the control proteins M2 pyruvate kinase, beta-tubulin and the cytokine inducible ICAM-1 was detected at low concentration in ibuprofen treated cells. The data demonstrate a possible mechanism how ibuprofen may decrease the risk and delay the onset of AD.
PMID:11741404 Blasko I et al; Neurobiol Dis 8 (6): 1094-101 (2001)
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2014-06-13
Pay. Date : 2014-06-09
DMF Number : 22431
Submission : 2009-01-15
Status : Active
Type : II

Certificate Number : R1-CEP 2008-316 - Rev 05
Issue Date : 2022-07-27
Type : Chemical
Substance Number : 721
Status : Valid

Date of Issue : 2022-03-24
Valid Till : 2025-03-17
Written Confirmation Number : WC-0004
Address of the Firm :

NDC Package Code : 49716-325
Start Marketing Date : 2017-06-15
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Sanil Pharmaceutical Co., Ltd.
Registration Date : 2023-05-24
Registration Number : 20210727-209-J-555(A)
Manufacturer Name : IOL Chemicals and Pharmaceuticals Limited
Manufacturer Address : Village: Fatebgarh Channa, Mansa Road (Trident Complex) Bamala-148101(Punjab) India
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 ASolution Pharmaceuticals: Your Partner for cGMP Development & Manufacturing Services – Supplying APIs, FDFs, and Specialty Molecules.
ASolution Pharmaceuticals: Your Partner for cGMP Development & Manufacturing Services – Supplying APIs, FDFs, and Specialty Molecules.

Date of Issue : 2024-01-23
Valid Till : 2026-06-17
Written Confirmation Number : WC-476
Address of the Firm :
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2014-06-13
Pay. Date : 2014-06-09
DMF Number : 22431
Submission : 2009-01-15
Status : Active
Type : II
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20606
Submission : 2007-06-12
Status : Active
Type : II
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35886
Submission : 2021-05-18
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4329
Submission : 1981-10-19
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4644
Submission : 1982-09-02
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4594
Submission : 1982-05-17
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2720
Submission : 1976-08-16
Status : Inactive
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2907
Submission : 1977-04-28
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2510
Submission : 1975-08-28
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2078
Submission : 1973-03-02
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
27
PharmaCompass offers a list of Ibuprofen API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ibuprofen manufacturer or Ibuprofen supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ibuprofen manufacturer or Ibuprofen supplier.
PharmaCompass also assists you with knowing the Ibuprofen API Price utilized in the formulation of products. Ibuprofen API Price is not always fixed or binding as the Ibuprofen Price is obtained through a variety of data sources. The Ibuprofen Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A p-Isobutylhydratropic acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of p-Isobutylhydratropic acid, including repackagers and relabelers. The FDA regulates p-Isobutylhydratropic acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. p-Isobutylhydratropic acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of p-Isobutylhydratropic acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A p-Isobutylhydratropic acid supplier is an individual or a company that provides p-Isobutylhydratropic acid active pharmaceutical ingredient (API) or p-Isobutylhydratropic acid finished formulations upon request. The p-Isobutylhydratropic acid suppliers may include p-Isobutylhydratropic acid API manufacturers, exporters, distributors and traders.
click here to find a list of p-Isobutylhydratropic acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A p-Isobutylhydratropic acid DMF (Drug Master File) is a document detailing the whole manufacturing process of p-Isobutylhydratropic acid active pharmaceutical ingredient (API) in detail. Different forms of p-Isobutylhydratropic acid DMFs exist exist since differing nations have different regulations, such as p-Isobutylhydratropic acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A p-Isobutylhydratropic acid DMF submitted to regulatory agencies in the US is known as a USDMF. p-Isobutylhydratropic acid USDMF includes data on p-Isobutylhydratropic acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The p-Isobutylhydratropic acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of p-Isobutylhydratropic acid suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The p-Isobutylhydratropic acid Drug Master File in Japan (p-Isobutylhydratropic acid JDMF) empowers p-Isobutylhydratropic acid API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the p-Isobutylhydratropic acid JDMF during the approval evaluation for pharmaceutical products. At the time of p-Isobutylhydratropic acid JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of p-Isobutylhydratropic acid suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a p-Isobutylhydratropic acid Drug Master File in Korea (p-Isobutylhydratropic acid KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of p-Isobutylhydratropic acid. The MFDS reviews the p-Isobutylhydratropic acid KDMF as part of the drug registration process and uses the information provided in the p-Isobutylhydratropic acid KDMF to evaluate the safety and efficacy of the drug.
After submitting a p-Isobutylhydratropic acid KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their p-Isobutylhydratropic acid API can apply through the Korea Drug Master File (KDMF).
click here to find a list of p-Isobutylhydratropic acid suppliers with KDMF on PharmaCompass.
A p-Isobutylhydratropic acid CEP of the European Pharmacopoeia monograph is often referred to as a p-Isobutylhydratropic acid Certificate of Suitability (COS). The purpose of a p-Isobutylhydratropic acid CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of p-Isobutylhydratropic acid EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of p-Isobutylhydratropic acid to their clients by showing that a p-Isobutylhydratropic acid CEP has been issued for it. The manufacturer submits a p-Isobutylhydratropic acid CEP (COS) as part of the market authorization procedure, and it takes on the role of a p-Isobutylhydratropic acid CEP holder for the record. Additionally, the data presented in the p-Isobutylhydratropic acid CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the p-Isobutylhydratropic acid DMF.
A p-Isobutylhydratropic acid CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. p-Isobutylhydratropic acid CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of p-Isobutylhydratropic acid suppliers with CEP (COS) on PharmaCompass.
A p-Isobutylhydratropic acid written confirmation (p-Isobutylhydratropic acid WC) is an official document issued by a regulatory agency to a p-Isobutylhydratropic acid manufacturer, verifying that the manufacturing facility of a p-Isobutylhydratropic acid active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting p-Isobutylhydratropic acid APIs or p-Isobutylhydratropic acid finished pharmaceutical products to another nation, regulatory agencies frequently require a p-Isobutylhydratropic acid WC (written confirmation) as part of the regulatory process.
click here to find a list of p-Isobutylhydratropic acid suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing p-Isobutylhydratropic acid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for p-Isobutylhydratropic acid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture p-Isobutylhydratropic acid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain p-Isobutylhydratropic acid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a p-Isobutylhydratropic acid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of p-Isobutylhydratropic acid suppliers with NDC on PharmaCompass.
p-Isobutylhydratropic acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of p-Isobutylhydratropic acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right p-Isobutylhydratropic acid GMP manufacturer or p-Isobutylhydratropic acid GMP API supplier for your needs.
A p-Isobutylhydratropic acid CoA (Certificate of Analysis) is a formal document that attests to p-Isobutylhydratropic acid's compliance with p-Isobutylhydratropic acid specifications and serves as a tool for batch-level quality control.
p-Isobutylhydratropic acid CoA mostly includes findings from lab analyses of a specific batch. For each p-Isobutylhydratropic acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
p-Isobutylhydratropic acid may be tested according to a variety of international standards, such as European Pharmacopoeia (p-Isobutylhydratropic acid EP), p-Isobutylhydratropic acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (p-Isobutylhydratropic acid USP).
