
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
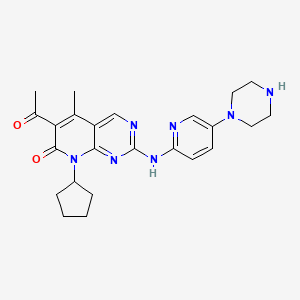

1. 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8h-pyrido(2,3-d)pyrimidin-7-one
2. Ibrance
3. Pd 0332991
4. Pd-0332991
5. Pd0332991
1. 571190-30-2
2. Pd-0332991
3. Ibrance
4. Pd0332991
5. Pd 0332991
6. Palbociclib Free Base
7. Unii-g9zf61le7g
8. 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[2,3-d]pyrimidin-7(8h)-one
9. Pd-332991
10. 571190-30-2 (free Base)
11. Mfcd11840850
12. 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7(8h)-one
13. 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8h-pyrido[2,3-d]pyrimidin-7-one
14. G9zf61le7g
15. Palbociclib(pd0332991)
16. Pd 332991
17. 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8h-pyrido(2,3-d)pyrimidin-7-one
18. Chebi:85993
19. 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one
20. Lqq
21. 2euf
22. 6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7(8h)-one
23. Pyrido(2,3-d)pyrimidin-7(8h)-one, 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(1-piperazinyl)-2-pyridinyl)amino)-
24. Palbociclib [usan]
25. 571190-30-2 Pound Not827022-32-2
26. Palbociclib [usan:inn]
27. [d8]-palbociclib
28. 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-8h-pyrido(2,3-d)pyrimidin-7-one
29. Ibrance (tn)
30. Palbociclib- Bio-x
31. Kinome_3823
32. Kinome_3824
33. Palbociclib [mi]
34. 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8h-pyrido[2,3-d]pyrimidin-7-one Hydrochloride
35. Palbociclib [inn]
36. Palbociclib [jan]
37. Palbociclib (jan/usan)
38. Palbociclib [who-dd]
39. Schembl462630
40. Bdbm6309
41. Chembl189963
42. Gtpl7380
43. Pd 0332991 (palbociclib)
44. Dtxsid40972590
45. Ex-a408
46. Palbociclib [orange Book]
47. 2euf; Pd 0332991
48. Otava-bb 1115529
49. Bcpp000125
50. Hms3265m09
51. Hms3265m10
52. Hms3265n09
53. Hms3265n10
54. Hms3744g13
55. Amy14886
56. Bcp09274
57. Bcp18381
58. Zinc3938686
59. Nsc758247
60. Nsc772256
61. Nsc800815
62. S4482
63. Akos022205241
64. Bcp9001058
65. Db09073
66. Nsc-758247
67. Nsc-772256
68. Nsc-800815
69. Sb40426
70. Pyrido-[2,3-d]-pyrimidin-7-one 43
71. Ncgc00263129-01
72. Ncgc00263129-08
73. Ncgc00263129-21
74. Ncgc00263129-22
75. Ac-25485
76. As-17016
77. Bp166224
78. Hy-50767
79. Sy026143
80. Ft-0697059
81. A14427
82. D10372
83. 190p302
84. Pd 0332991,pd0332991
85. Pd-0332991, Pd0332991
86. Brd-k51313569-001-01-1
87. P-0332991
88. Q15269707
89. 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino(pyrido(2,3-d)pyrimidin-7(8h)-one
90. 6-acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-8h-pyrido[2,3-d]pyrimidin-7-one
91. 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[6,5-d]pyrimidin-7-one
92. 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyrido[2,3-d]pyrimidin-7(8h)-one
93. 6-acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)-pyridin-2-yl]amino]-8h-pyrido[2,3-d]pyrimidin-7-one
94. 8-cyclopentyl-6-acetyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7h,8h-pyrido[2,3-d]pyrimidin-7-one
| Molecular Weight | 447.5 g/mol |
|---|---|
| Molecular Formula | C24H29N7O2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 447.23827319 g/mol |
| Monoisotopic Mass | 447.23827319 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 775 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Palbociclib is indicated in combination with [letrozole] as initial endocrine-based therapy for the treatment of human epidermal growth factor receptor type 2 (HER2)-negative and hormone receptor(HR)-positive tumors in adult patients with advanced/metastatic breast cancer. It is as well approved in combination with [fulvestrant] in patients with disease progression with prior endocrine therapy. In the official labeling, the use of palbociclib should be accompanied with either an aromatase inhibition, no restricted to letrozole, as initial endocrine-based therapy in postmenopausal women or in man. The breast cancer starts as a group of cancer cells that grow into and destroy the nearby breast tissue. This growth can spread into other parts of the body which is called metastasis. According to the location of the cancer cells, it can be categorized in ductal carcinoma and lobular carcinoma. However, other types of breast cancer include inflammatory breast cancer, Paget disease of the breast, triple negative breast cancer non-Hodgkin lymphoma and soft tissue sarcoma. In males, breast cancer is usually treated as the cases of postmenopausal women and almost all the cases are ductal carcinoma.
FDA Label
Ibrance is indicated for the treatment of hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative locally advanced or metastatic breast cancer :
- in combination with an aromatase inhibitor;
- in combination with fulvestrant in women who have received prior endocrine therapy.
In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinizing hormone releasing hormone (LHRH) agonist.
Treatment of breast malignant neoplasms
Treatment of Ewing sarcoma
Due to its mechanism of action, palbociclib inhibits cell growth and suppresses DNA replication in retinoblastoma tumor suppressor gene (RB) proficient cancer cells. As expected, these RB cells present a significant increase in the proportion of cells in G1 state and the presence of palbociclib produces effective dephosphorylation of RB, reduce proliferation and induce senescence causing cell-cycle arrest. In vitro studies showed the potential for palbociclib to reduce cellular proliferation of estrogen receptor-positive breast cancer cell lines through the inhibition of the cell-cycle progression from G1 to S phase. In this study, it was demonstrated that the sensitivity of the cells significantly increased with the expression of _RB1_ and _CCND1_ and low expression of _CDKN2A_. As well, palbociclib, combined with antiestrogens, enhanced _in vivo_ antitumor activity in estrogen receptor-positive breast cancer mouse models. In clinical trials, palbociclib, in combination with letrozole, was shown to significantly increase the progression-free survival (PFS) in patients with metastatic breast cancer without prior endocrine treatment. In the results, the PFS increased from 4.5 to 9.5 months with an overall response rate (ORR) of 24.6%.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE33
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EF - Cyclin-dependent kinase (cdk) inhibitors
L01EF01 - Palbociclib
Absorption
Palbociclib presents a linear pharmacokinetic profile and its peak plasma concentration was observed 6-12 hours after oral administration. The oral bioavailability is reported to be of 46% with a steady-state reached after 8 days and a median accumulation ratio of 2.4. The absorption of palbociclib is significantly reduced under fasting conditions and hence, food intake is recommended when this drug is administered.
Route of Elimination
The main route of elimination of palbociclib is through feces after hepatic metabolism while renal clearance seems to play a minor role accounting only for 17.5% of the eliminated dose.
Volume of Distribution
The mean apparent distribution of palbociclib is 2583 L which suggests that palbociclib penetrates extensively into peripheral tissues.
Clearance
The mean apparent oral clearance of palbociclib is of 63.1 L/h.
Palbociclib is mainly hepatically transformed. the metabolism is mainly performed by the activities of the cytochrome P450 isoenzyme 3A and the sulfotransferase 2A1. The metabolism of palbociclib is represented mainly by reactions of oxidation and sulfonation followed by acylation and glucuronidation as minor reactions. After its metabolism, palbociclib forms mainly inactive glucuronide and sulfamic acid conjugates. The major circulating metabolite, accounting for 1.5% of the dose in excreta is is the glucuronide conjugate.
The mean plasma elimination half-life of palbociclib is 29 hours.
Palbociclib is a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor that acts by binding to the ATP pocket with an IC50 in the range of 9-15 nmol/L. It is important to consider that it presents low to absent activity against other kinases. The CDK4/6 kinase is involved, with coregulatory partner cyclin D, in the G1-S transition. Hence, inhibition of this step prevents cell cycle progression in cells in whose this pathway is functioning. This step includes the pathways of the phosphorylation of retinoblastoma protein and the E2F family of transcription factors.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
76
PharmaCompass offers a list of Palbociclib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Palbociclib manufacturer or Palbociclib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Palbociclib manufacturer or Palbociclib supplier.
PharmaCompass also assists you with knowing the Palbociclib API Price utilized in the formulation of products. Palbociclib API Price is not always fixed or binding as the Palbociclib Price is obtained through a variety of data sources. The Palbociclib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Palbociclib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Palbociclib, including repackagers and relabelers. The FDA regulates Palbociclib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Palbociclib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Palbociclib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Palbociclib supplier is an individual or a company that provides Palbociclib active pharmaceutical ingredient (API) or Palbociclib finished formulations upon request. The Palbociclib suppliers may include Palbociclib API manufacturers, exporters, distributors and traders.
click here to find a list of Palbociclib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Palbociclib DMF (Drug Master File) is a document detailing the whole manufacturing process of Palbociclib active pharmaceutical ingredient (API) in detail. Different forms of Palbociclib DMFs exist exist since differing nations have different regulations, such as Palbociclib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Palbociclib DMF submitted to regulatory agencies in the US is known as a USDMF. Palbociclib USDMF includes data on Palbociclib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Palbociclib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Palbociclib suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Palbociclib Drug Master File in Korea (Palbociclib KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Palbociclib. The MFDS reviews the Palbociclib KDMF as part of the drug registration process and uses the information provided in the Palbociclib KDMF to evaluate the safety and efficacy of the drug.
After submitting a Palbociclib KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Palbociclib API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Palbociclib suppliers with KDMF on PharmaCompass.
A Palbociclib written confirmation (Palbociclib WC) is an official document issued by a regulatory agency to a Palbociclib manufacturer, verifying that the manufacturing facility of a Palbociclib active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Palbociclib APIs or Palbociclib finished pharmaceutical products to another nation, regulatory agencies frequently require a Palbociclib WC (written confirmation) as part of the regulatory process.
click here to find a list of Palbociclib suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Palbociclib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Palbociclib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Palbociclib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Palbociclib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Palbociclib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Palbociclib suppliers with NDC on PharmaCompass.
Palbociclib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Palbociclib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Palbociclib GMP manufacturer or Palbociclib GMP API supplier for your needs.
A Palbociclib CoA (Certificate of Analysis) is a formal document that attests to Palbociclib's compliance with Palbociclib specifications and serves as a tool for batch-level quality control.
Palbociclib CoA mostly includes findings from lab analyses of a specific batch. For each Palbociclib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Palbociclib may be tested according to a variety of international standards, such as European Pharmacopoeia (Palbociclib EP), Palbociclib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Palbociclib USP).
