

Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Finished Drug Prices
NA

1. Cotazym S
2. Cotazym-s
3. Cotazyme
4. Creon
5. Encron
6. Ilozyme
7. Ku Zyme
8. Ku-zyme
9. Lipram
10. Pancrease
11. Pancrecarb
12. Pancrelipase
13. Pancron
14. Panokase
15. Pertzye
16. Protilase
17. Ultrase
18. Viokase
19. Zymase
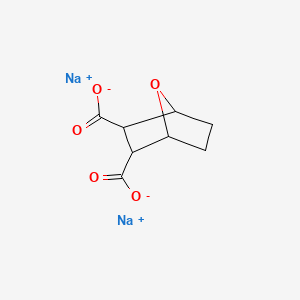
1. 13114-29-9
2. Sodium Demethylcantharidate
3. 129-67-9
4. Sodium 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate
5. Disodium;7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylate
6. Disodium Endothal
7. Sodium 3-carboxy-7-oxabicyclo[2.2.1]heptane-2-carboxylate
8. Pancrelipase
9. Sodium Norcantharidin
10. Endothall-sodium
11. Sodium-demethylcantharidate
12. 53608-75-6
13. Schembl1006891
14. Dtxsid1041899
15. Chebi:81916
16. Ft-0775390
17. C18724
18. Q27155657
19. Sodium3-carboxy-7-oxabicyclo[2.2.1]heptane-2-carboxylate
| Molecular Weight | 230.13 g/mol |
|---|---|
| Molecular Formula | C8H8Na2O5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 230.01671192 g/mol |
| Monoisotopic Mass | 230.01671192 g/mol |
| Topological Polar Surface Area | 89.5 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 224 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
The use of pancrelipase amylase is part of the pancreatic enzyme replacement therapy. This therapy is indicated for the treatment of pancreatic insufficiency attributed to cystic fibrosis, chronic pancreatitis or any other medically defined pancreatic disease that might require it. Pancreatic diseases are associated with the deterioration of pancreatic parenchyma and of the dual physiological functions of the pancreas. Once established, pancreatic insufficiency results in malnutrition, weight loss, and steatorrhea.
FDA Label
The major maldigestion/malabsorption problems arise from incomplete fat digestion. In clinical trials, the administration of pancrelipase as a mixture of amylase, lipase, and protease demonstrated a significant improvement in the coefficient of fat absorption and nitrogen absorption. These effects are accompanied by increased in body weight and body mass index.
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
Absorption
Pancrelipase acts locally in the GI tract and it is not absorbed in any significant amount.
Route of Elimination
Pancrelipase is entirely eliminated in the feces.
Volume of Distribution
Pancrelipase acts locally in the GI tract and it is not absorbed in any significant amount thus, the volume of distribution is not relevant.
Clearance
Pancrelipase acts locally in the GI tract and it is not absorbed in any significant amount thus, the clearance rate is not relevant.
Pancrelipase acts locally in the GI tract and it is not absorbed in any significant amount thus, the metabolism is not relevant.
Pancrelipase acts locally in the GI tract and it is not absorbed in any significant amount thus, the elimination half-life is not relevant.
Pancrelipase is used to replace the deficiency of pancreatic enzymes. As abovementioned, pancrelipase is formed by a mixture of lipase, protease, and amylase which are able to break down fat, protein, and starches, respectively, in the small intestine. For a more specific description of each mechanism of action, please visit [DB11065], [DB11066] and [DB13147].
 ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7090
Submission : 1987-07-28
Status : Active
Type : II

Certificate Number : R1-CEP 2001-280 - Rev 03
Issue Date : 2021-04-01
Type : Chemical
Substance Number : 350
Status : Valid

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9883
Submission : 1992-09-25
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Certificate Number : R1-CEP 2001-215 - Rev 02
Issue Date : 2010-11-26
Type : Chemical
Substance Number : 350
Status : Withdrawn by Holder

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
NDC Package Code : 31387-219
Start Marketing Date : 2004-02-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 31387-205
Start Marketing Date : 2004-02-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 31387-137
Start Marketing Date : 2004-02-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 31387-165
Start Marketing Date : 2007-06-21
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 51421-001
Start Marketing Date : 2022-07-22
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
ChemWerth works in generic API development & supply, non-infringement patent strategy development and regulatory support.
About the Company : Established in 1982, ChemWerth is a US-headquartered full-service generic active pharmaceutical ingredient (API) development and supply company. ChemWerth offers cGMP-quality APIs ...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Reply
03 Apr 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
