
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
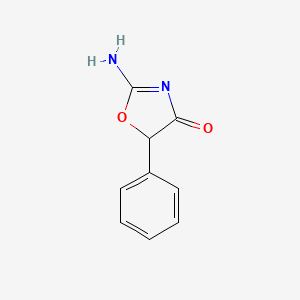

1. Compounds, Pemoline
2. Cylert
3. Magnesium, Pemoline
4. Pemadd
5. Pemoline Compounds
6. Pemoline Magnesium
7. Phenoxazole
8. Phenylisohydantoin
9. Tradon
1. Phenoxazole
2. Phenylisohydantoin
3. Azoksodon
4. Fenoxazol
5. Cylert
6. Tradon
7. Dantromin
8. Azoxodon
9. Pemolin
10. 2152-34-3
11. Azoxodone
12. Centramin
13. Deltamine
14. Hyton
15. Nitan
16. Kethamed
17. Sigmadyn
18. Stimulol
19. Okodon
20. Pioxol
21. Pondex
22. Sistra
23. Stimul
24. Tradone
25. Volital
26. Ronyl
27. Phenylpseudohydantoin
28. Pheniminooxazolidinone
29. Betanamin
30. Deltamin
31. Myamin
32. Constimol
33. Phenalone
34. Phenilone
35. Pomoline
36. Endolin
37. Notair
38. Volitol
39. Hyton Asa
40. Juston-wirkstoff
41. Pemolina
42. Sistral
43. Senior
44. 2-imino-5-phenyl-4-oxazolidinone
45. Yh 1
46. 4(5h)-oxazolone, 2-amino-5-phenyl-
47. Npl 1
48. 2-amino-5-phenyl-4(5h)-oxazolone
49. 2-amino-5-phenyl-1,3-oxazol-4(5h)-one
50. 2-amino-5-phenyl-1,3-oxazol-4-one
51. Pemolinum
52. Abbott 13397
53. 5-phenyl-2-imino-4-oxazolidinone
54. 5-phenyl-2-imino-4-oxooxazolidine
55. 4-oxazolidinone, 2-imino-5-phenyl-
56. Nsc-25159
57. Chebi:7953
58. 2-imino-4-keto-5-phenyltetrahydrooxazole
59. 2-amino-5-phenyl-oxazol-4-one
60. Pn/135
61. Fwh-352
62. La 956
63. Pt 360
64. 101053-01-4
65. Nsc-169499
66. Yh-1
67. Pio
68. H 310
69. C- 293
70. A 13397
71. La-956
72. Fio
73. 2-oxazolin-4-one, 2-amino-5-phenyl-
74. Dsstox_cid_3427
75. Dsstox_rid_81815
76. Dsstox_gsid_23427
77. Wln: T5oymv Ehj Bum Er
78. 5-phenyl-2-imino-4-oxazolidine
79. P 10
80. Smr000238142
81. Cas-2152-34-3
82. 2-amino-5-phenyl4(5h)-oxazolone
83. Cylert (tn)
84. Pemoline [hsdb]
85. Pemoline [usan]
86. Pemoline [inn]
87. Pemoline [jan]
88. Pemoline [mi]
89. Pemoline [vandf]
90. Pemoline [mart.]
91. Pemoline [who-dd]
92. Chembl1177
93. Schembl41636
94. Pemoline (jan/usan/inn)
95. Mls000759491
96. Mls001424026
97. Pemoline [orange Book]
98. 2-amino-5-phenyl-4-oxazolone
99. Pemoline, >=98% (hplc)
100. Nrncyvbfpddjne-uhfffaoysa-
101. Hms2051c08
102. Hms3393c08
103. Nsc25159
104. 5-phenyl-2-imino-4-oxo-oxazolidin
105. Tox21_112509
106. 2-amino-5-phenyl-2-oxazolin-4-one
107. Bdbm50248019
108. Nsc169499
109. Akos005065730
110. Akos015888248
111. Tox21_112509_1
112. 2-azanyl-5-phenyl-1,3-oxazol-4-one
113. Ccg-100848
114. Db01230
115. Nc00098
116. Ncgc00246967-01
117. Ncgc00246967-02
118. 5-phenyl-2-iminooxazolidin-4-one
119. Ac-22513
120. As-17043
121. 2-imino-4-oxo-5-phenyloxazolidine
122. Ft-0655727
123. P0392
124. 2-amino-5-phenyl-1,3-oxazol-4(5h)-one #
125. C07899
126. D00744
127. A800319
128. A815446
129. W-107545
| Molecular Weight | 176.17 g/mol |
|---|---|
| Molecular Formula | C9H8N2O2 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 176.058577502 g/mol |
| Monoisotopic Mass | 176.058577502 g/mol |
| Topological Polar Surface Area | 64.7 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 244 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Central Nervous System Stimulants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The Agency has concluded that the overall risk of liver toxicity from Cylert and generic pemoline products outweighs the benefits of this drug. In May 2005, Abbott chose to stop sales and marketing of Cylert in the U.S. All generic companies have also agreed to stop sales and marketing of this product (Pemoline tablets and chewable tablets). Cylert is a central nervous system stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). This product is considered second line therapy for ADHD because of its association with life threatening hepatic failure.
FDA; Center for Drug Evaluation and Research; Alert for Healthcare Professionals, Pemoline Tablets and Chewable Tablets (marketed as Cylert) (October 2005). Available from, as of January 15, 2008: https://www.fda.gov/cder/drug/InfoSheets/HCP/pemolineHCP.htm
Adjunct to psychological, educational, social, and other remedial measures in the treatment of attention deficit disorder with hyperactivity (hyperkinetic syndrome of childhood, minimal brain dysfunction) in carefully selected children older than 6 years of age. /Use is included in the labeling approved by the US Food and Drug Administration/.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1309
Pemoline has been used in the treatment of fatigue, mental depressions, and chronic schizophrenia, and as a mild stimulant for geriatric patients. /Uses are not included in the labeling approved by the US Food and Drug Administration/.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1309
The Agency has concluded that the overall risk of liver toxicity from Cylert and generic pemoline products outweighs the benefits of this drug. In May 2005, Abbott chose to stop sales and marketing of Cylert in the U.S. All generic companies have also agreed to stop sales and marketing of this product (Pemoline tablets and chewable tablets). Cylert is a central nervous system stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). This product is considered second line therapy for ADHD because of its association with life threatening hepatic failure.
FDA; Center for Drug Evaluation and Research; Alert for Healthcare Professionals, Pemoline Tablets and Chewable Tablets (marketed as Cylert) (October 2005). Available from, as of January 15, 2008: https://www.fda.gov/cder/drug/InfoSheets/HCP/pemolineHCP.htm
Because of its association with life threatening hepatic failure, Cylert should not ordinarily be considered as first line drug therapy for ADHD. Because Cylert provides an observable symptomatic benefit, patients who fail to show substantial clinical benefit within 3 weeks of completing dose titration, should be withdrawn from Cylert therapy.
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
Treatment with Cylert should be initiated only in individuals without liver disease and with normal baseline liver function tests. It is not clear if baseline and periodic liver function testing are predictive of these instances of acute liver failure; however, it is generally believed that early detection of drug induced hepatic injury along with immediate withdrawal of the suspect drug enhances the likelihood for recovery. Accordingly, the following liver monitoring program is recommended: Serum ALT (SGPT) levels should be determined at baseline, and every two weeks thereafter. If Cylert therapy is discontinued and then restarted, liver function test monitoring should be done at baseline and reinitiated at the frequency above. Cylert should be discontinued if serum ALT (SGPT) is increased to a clinically significant level, or any increase > or = 2 times the upper limit of normal, or if clinical signs and symptoms suggest liver failure
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
Clinical experience suggests that in psychotic children, administration of Cylert may exacerbate symptoms of behavior disturbance and thought disorder.
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
For more Drug Warnings (Complete) data for PEMOLINE (16 total), please visit the HSDB record page.
For treatment of Attention Deficit Hyperactivity Disorder (ADHD)
FDA Label
Pemoline belongs to the group of medicines called central nervous system (CNS) stimulants. It is used to treat attention deficit hyperactivity disorder (ADHD). Pemoline stimulates the brain, probably by affecting neurotransmitters, the chemicals in the brain that nerves use to communicate with each other.
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BA - Centrally acting sympathomimetics
N06BA05 - Pemoline
Absorption
Pemoline is rapidly absorbed from the gastrointestinal tract
Route of Elimination
Pemoline is excreted primarily by the kidneys with approximately 50% excreted unchanged and only minor fractions present as metabolites.
Pemoline is rapidly absorbed from the gastrointestinal tract. Approximately 50% is bound to plasma proteins. The serum half-life of pemoline is approximately 12 hours. Peak serum levels of the drug occur within 2 to 4 hours after ingestion of a single dose. Multiple dose studies in adults at several dose levels indicate that steady state is reached in approximately 2 to 3 days. In animals given radiolabeled pemoline, the drug was widely and uniformly distributed throughout the tissues, including the brain.
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
... Pemoline and its metabolites are excreted primarily in urine; only negligible amounts are excreted in feces. About 75% of an oral dose is excreted in urine within 24 hr; about 43% is excreted unchanged and about 22% is excreted as pemoline conjugates.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1309
Pemoline is absorbed from the GI tract, and peak serum concentrations are achieved within 2-4 hours. Multiple-dose studies in adults indicate that serum concentrations plateau in about 3 days. In a study involving adults, the CNS stimulant effect of a single oral dose of pemoline was relatively long, reaching its peak within 4 hr and lasting at least 8 hr. However, when pemoline is administered to children in the treatment of attention deficit disorder, the drug has a gradual onset of action and therapeutic effects may not be apparent until 2 or 3 weeks of therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1308
Hepatic
Pemoline is metabolized by the liver. Metabolites of pemoline include pemoline conjugate, pemoline dione, mandelic acid, and unidentified polar compounds. Cylert is excreted primarily by the kidneys with approximately 50% excreted unchanged and only minor fractions present as metabolites.
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
The serum half-life of pemoline is approximately 12 hours.
The serum half-life of pemoline is approximately 12 hours.
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
Following a single oral dose in healthy adults, the plasma or serum half-life of pemoline has ranged from about 9-14 hr. Following a single oral dose in children, the plasma elimination half-life exhibits considerable interindividual variation, ranging from about 2-12 hr (mean: 8.6 hr). Preliminary evidence suggests that the drug may exhibit nonlinar kinetics in children following multiple dosing, with the elimination half-life increasing substantially. ...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1309
The pharmacologic actions of pemoline are qualitatively similar to those of the amphetamines and methylphenidate, and include CNS and respiratory stimulation and weak sympathomimetic activity. ... Limited animal experiments suggest that the CNS stimulatory action of pemoline may be mediated by brain dopamine. Pemoline may produce an increase in motor activity, mental alertness, diminished sense of fatigue, and mild euphoria. ... In usual therapeutic dosage, pemoline exhibits no substantial effects on the peripheral circulatory system.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1309
Cylert (pemoline) has a pharmacological activity similar to that of other known central nervous system stimulants; however, it has minimal sympathomimetic effects. Although studies indicate that pemoline may act in animals through dopaminergic mechanisms, the exact mechanism and site of action of the drug in man is not known.
FDA; Center for Drug Evaluation and Research; Label Information for Cylert (Pemoline) (Last updated December 2002). Available from, as of January 15, 2008: https://www.fda.gov/cder/foi/label/2003/016832s022_017703s018lbl.pdf
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
85
PharmaCompass offers a list of Pemoline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pemoline manufacturer or Pemoline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pemoline manufacturer or Pemoline supplier.
PharmaCompass also assists you with knowing the Pemoline API Price utilized in the formulation of products. Pemoline API Price is not always fixed or binding as the Pemoline Price is obtained through a variety of data sources. The Pemoline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pemoline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pemoline, including repackagers and relabelers. The FDA regulates Pemoline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pemoline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pemoline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pemoline supplier is an individual or a company that provides Pemoline active pharmaceutical ingredient (API) or Pemoline finished formulations upon request. The Pemoline suppliers may include Pemoline API manufacturers, exporters, distributors and traders.
click here to find a list of Pemoline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pemoline DMF (Drug Master File) is a document detailing the whole manufacturing process of Pemoline active pharmaceutical ingredient (API) in detail. Different forms of Pemoline DMFs exist exist since differing nations have different regulations, such as Pemoline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pemoline DMF submitted to regulatory agencies in the US is known as a USDMF. Pemoline USDMF includes data on Pemoline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pemoline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pemoline suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Pemoline Drug Master File in Japan (Pemoline JDMF) empowers Pemoline API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Pemoline JDMF during the approval evaluation for pharmaceutical products. At the time of Pemoline JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Pemoline suppliers with JDMF on PharmaCompass.
Pemoline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pemoline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pemoline GMP manufacturer or Pemoline GMP API supplier for your needs.
A Pemoline CoA (Certificate of Analysis) is a formal document that attests to Pemoline's compliance with Pemoline specifications and serves as a tool for batch-level quality control.
Pemoline CoA mostly includes findings from lab analyses of a specific batch. For each Pemoline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pemoline may be tested according to a variety of international standards, such as European Pharmacopoeia (Pemoline EP), Pemoline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pemoline USP).
