
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Australia
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
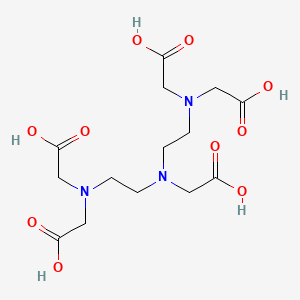

1. Ca-dtpa
2. Cadtpa
3. Calcium Trisodium Pentetate
4. Cana-dtpa
5. Detapac
6. Diethylenetriamine Pentaacetic Acid
7. Dtpa
8. Indium Dtpa
9. Indium-dtpa
10. Mn-dtpa
11. Pentaacetic Acid, Diethylenetriamine
12. Pentacin
13. Pentacine
14. Pentaind
15. Pentetate Calcium Trisodium
16. Pentetate Zinc Trisodium
17. Pentetate, Calcium Trisodium
18. Pentetates
19. Penthanil
20. Sn-dtpa
21. Zinc Dtpa
22. Zinc-dtpa
1. Diethylenetriaminepentaacetic Acid
2. 67-43-6
3. Dtpa
4. Detapac
5. Detarex
6. Complexon V
7. Titriplex V
8. Perma Kleer
9. Monaquest Cai
10. Hamp-ex Acid
11. Penthanil
12. Dabeersen 503
13. Chel 330 Acid
14. Diethylenetriamine Pentaacetic Acid
15. Pentacarboxymethyldiethylenetriamine
16. Chel Dtpa
17. Pentetate
18. Penthamil
19. Acidum Penteticum
20. H5dtpa
21. (diethylenetrinitrilo)pentaacetic Acid
22. Diethylenetriamine-n,n,n',n'',n''-pentaacetic Acid
23. Detpa
24. 2-[bis[2-[bis(carboxymethyl)amino]ethyl]amino]acetic Acid
25. Glycine, N,n-bis(2-(bis(carboxymethyl)amino)ethyl)-
26. Monaquest
27. 1,1,4,7,7-diethylenetriaminepentaacetic Acid
28. Dtp-a
29. N,n-bis{2-[bis(carboxymethyl)amino]ethyl}glycine
30. Diethylene Triamine Pentaacetic Acid
31. Nsc 7340
32. Glycine, N,n-bis[2-[bis(carboxymethyl)amino]ethyl]-
33. Diethylenetriaminepentacetic Acid
34. Nsc-7340
35. 3,6,9-triazaundecanedioic Acid, 3,6,9-tris(carboxymethyl)-
36. Mfcd00004289
37. N,n-bis[2-[bis(carboxymethyl)amino]ethyl]glycine
38. 7a314hqm0i
39. Chel 330
40. Diethylenetriaminepentaaceticacid
41. Acetic Acid, ((carboxymethylimino)bis(ethylenenitrilo))tetra-
42. Chebi:35739
43. Nsc7340
44. Diethylenetriaminepentaacetate
45. (diethylenetriamine)pentaacetic Acid
46. N,n-bis(2-(bis(carboxymethyl)amino)ethyl)glycine
47. N,n-bis(2-(bis-(carboxymethyl)amino)ethyl)-glycine
48. Acetic Acid, 2,2',2'',2'''-(((carboxymethyl)imino)bis(2,1-ethanediylnitrilo))tetrakis-
49. Diethylenetriaminepentaacetic Acid;dtpa
50. Cas-67-43-6
51. Ncgc00015360-05
52. (((carboxymethyl)imino)bis(ethylenenitrilo))tetraacetic Acid
53. Dissolvine D
54. Dsstox_cid_3434
55. Dsstox_rid_77025
56. Dsstox_gsid_23434
57. Penthamil (van)
58. N,n,n',n'',n''-diethylenetriaminepentaacetic Acid
59. [[(carboxymethyl)imino]bis(ethylenenitrilo)]tetraacetic Acid
60. 2-[bis({2-[bis(carboxymethyl)amino]ethyl})amino]acetic Acid
61. Acido Pentetico
62. Acide Pentetique
63. [[(carboxymethyl)imino]bis(1,2-ethanediylnitrilo)tetraacetic Acid]
64. 2,2',2'',2''',2''''-(ethane-1,2-diylnitrilo)pentaacetic Acid
65. Diethylenetriaminepentaacetic Acid, 99%
66. Acide Pentetique [inn-french]
67. Acido Pentetico [inn-spanish]
68. Acidum Penteticum [inn-latin]
69. Sr-01000075826
70. Einecs 200-652-8
71. Brn 1810219
72. Unii-7a314hqm0i
73. Acetic Acid, [(carboxymethylimino)bis(ethylenenitrilo)]tetra-
74. Pentetic-acid
75. N-carboxymethyliminobis(ethylenenitrilo)tetra(acetic Acid)
76. Acetic Acid, 2,2',2'',2'''-[[(carboxymethyl)imino]bis(2,1-ethanediylnitrilo)]tetrakis-
77. Pentetic Acid [usan:usp:inn:ban]
78. Nanodtpa
79. 2,2'-(carboxymethylimino)bis(ethyliminodiessigsaeure)
80. Pentetic Acid; Dtpa
81. Dtpa (pentetic Acid)
82. Plexene D (salt/mix)
83. Syntron C (salt/mix)
84. Kiresuto P (salt/mix)
85. Tetralon B (salt/mix)
86. Prestwick0_000941
87. Prestwick1_000941
88. Prestwick2_000941
89. Prestwick3_000941
90. Lopac-d-6518
91. Pentetate [vandf]
92. Chel 330 (salt/mix)
93. Chembl780
94. Ec 200-652-8
95. Pentetic Acid (usp/inn)
96. Pentetic Acid [ii]
97. Pentetic Acid [mi]
98. Pentetic Acid [inn]
99. Lopac0_000431
100. Schembl17138
101. Bspbio_000902
102. Pentetic Acid [inci]
103. Pentetic Acid [usan]
104. 4-04-00-02454 (beilstein Handbook Reference)
105. Nanodtpa Component Dtpa
106. Pentetic Acid [vandf]
107. Spbio_003061
108. Pentetic Acid [mart.]
109. Bpbio1_000994
110. Pentetic Acid [usp-rs]
111. Pentetic Acid [who-dd]
112. Dtxsid2023434
113. Hms1570n04
114. Hms2094e03
115. Hms2097n04
116. Hms3261g04
117. Hms3714n04
118. Pharmakon1600-01506082
119. Hy-b1335
120. Tox21_110131
121. Tox21_500431
122. Ac-333
123. Bbl002988
124. Nsc759314
125. S4824
126. Stk373226
127. Zinc19419017
128. Pentetic Acid [usp Monograph]
129. Wln: Qv1n1vq2n1vq2n1vq1vq
130. Akos005446652
131. Tox21_110131_1
132. Ccg-204523
133. Db14007
134. Lp00431
135. Nanodtpa Zn-dtpa Component Dtpa
136. Nsc-759314
137. Sdccgsbi-0050416.p003
138. Nano-dtpa Capsule Component Dtpa
139. Ncgc00015360-01
140. Ncgc00015360-02
141. Ncgc00015360-03
142. Ncgc00015360-04
143. Ncgc00015360-06
144. Ncgc00015360-08
145. Ncgc00015360-09
146. Ncgc00015360-12
147. Ncgc00093852-01
148. Ncgc00093852-02
149. Ncgc00093852-03
150. Ncgc00261116-01
151. Vs-01284
152. Sbi-0050416.p002
153. 1,4,7,7-diethylenetriaminepentaacetic Acid
154. Ab00375916
155. Cs-0013088
156. D0504
157. Eu-0100431
158. Ft-0624901
159. D 6518
160. D05422
161. Diethylenetriaminepentaacetic Acid, P.a., 99%
162. Ab00375916-04
163. Ab00375916_05
164. Diethylenetriamine Pentaacetic Acid [vandf]
165. Diethylenetriamine-n,n',n'',n''-pentaacetic Acid
166. Glycine,n-bis[2-[bis(carboxymethyl)amino]ethyl]-
167. Q416487
168. N,n-bis {2-[bis(carboxymethyl)amino]ethyl}glycine
169. Sr-01000075826-1
170. Sr-01000075826-4
171. Brd-k40621224-001-01-8
172. Diethylenetriaminepentaacetic Acid, >=98% (titration)
173. Diethylenetriaminepentaacetic Acid, >=99% (titration)
174. N, {n-bis[2-[bis(carboxymethyl)amino]ethyl]glycine}
175. Glycine, {n,n-bis[2-[bis(carboxymethyl)amino]ethyl]-}
176. 3,9-triazaundecanedioic Acid, 3,6,9-tris(carboxymethyl)-
177. Diethylenetriamine- N,n,n',n',n''-pentaacetic Acid
178. Dtpa, Diethylenetriamine-n,n,n',n'',n''-pentaacetic Acid
179. (((carboxymethyl)imino)bis(ethylenenitrilo))-tetraacetic Acid
180. {[[(carboxymethyl)imino]bis(ethylenenitrilo)]tetraacetic} Acid
181. Acetic Acid, {[(carboxymethylimino)bis(ethylenenitrilo)]tetra-}
182. Diethylenetriaminepentaacetic Acid, For Complexometry, >=99.0%
183. Pentetic Acid, United States Pharmacopeia (usp) Reference Standard
184. Acetic Acid,2',2'',2'''-[[(carboxymethyl)imino]bis(2,1-ethanediylnitrilo)]tetrakis-
185. 1004765-76-7
186. 2,2',2'',2'''-(2,2'-(carboxymethylazanediyl)bis(ethane-2,1-diyl)bis(azanetriyl))tetraacetic Acid
| Molecular Weight | 393.35 g/mol |
|---|---|
| Molecular Formula | C14H23N3O10 |
| XLogP3 | -8.5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 16 |
| Exact Mass | 393.13834394 g/mol |
| Monoisotopic Mass | 393.13834394 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 481 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | An-dtpa |
| PubMed Health | Technetium Tc 99m Pentetate (Injection) |
| Drug Classes | Diagnostic Agent, Radiopharmaceutical Imaging |
| Drug Label | Each kit consists of reaction vials which contain the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Pentetate Injection for diagnostic use by intravenous injection. Each 10 mL reaction vial contains 20 mg... |
| Active Ingredient | Technetium tc-99m pentetate kit |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Jubilant Draximage |
| 2 of 4 | |
|---|---|
| Drug Name | Dtpa |
| Active Ingredient | Technetium tc-99m pentetate kit |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Draximage |
| 3 of 4 | |
|---|---|
| Drug Name | An-dtpa |
| PubMed Health | Technetium Tc 99m Pentetate (Injection) |
| Drug Classes | Diagnostic Agent, Radiopharmaceutical Imaging |
| Drug Label | Each kit consists of reaction vials which contain the sterile, non-pyrogenic, non-radioactive ingredients necessary to produce Technetium Tc 99m Pentetate Injection for diagnostic use by intravenous injection. Each 10 mL reaction vial contains 20 mg... |
| Active Ingredient | Technetium tc-99m pentetate kit |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Jubilant Draximage |
| 4 of 4 | |
|---|---|
| Drug Name | Dtpa |
| Active Ingredient | Technetium tc-99m pentetate kit |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | n/a |
| Market Status | Prescription |
| Company | Draximage |
DTPA is widely used in industry and medicine. As a medical agent, it is approved for its use in medical imaging and for the decorporation of internally deposited radionuclides. It is FDA approved for the treatment of individuals with known or suspected internal contamination with plutonium, americium or curium to increase the rates of elimination. Due to the pharmacokinetic elimination by the kidneys, pentetic acid conjugated with technetium Tc-99m is being used clinically to estimate physiological parameters such as glomerular filtration rat and effective renal plasma flow.
FDA Label
There are reports in vivo of low stability of complexes of DPTA with uranium and neptunium which is being reported to cause deposition of the radionuclides into the tissues. In the case of plutonium, some preclinical studies have shown a very high urine elimination efficacy 1 hour after initial contamination. This efficacy is conserved for approximately 24 hours while the radiocontaminant is circulating. When the radionuclide is inhaled, it has been reported a DPTA-induced reduction of even 98% of the lung deposits. It is important to consider that pentetic acid can bind directly to other trace metals in the body which can cause deficiencies.
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
Iron Chelating Agents
Organic chemicals that form two or more coordination links with an iron ion. Once coordination has occurred, the complex formed is called a chelate. The iron-binding porphyrin group of hemoglobin is an example of a metal chelate found in biological systems. (See all compounds classified as Iron Chelating Agents.)
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Absorption
DTPA and its trisodium salts present a very poor bioavailability after oral administration. Therefore, the normal administration of DTPA is done by slow intravenous infusion or inhalation with a nebulizer. When inhaled, the absorption is of about 20% of the administered dose.
Route of Elimination
DTPA metal complexes are quickly excreted in the urine.It is predominantly excreted by the kidney and it is not excreted by non-renal routes to any significant extent.
Volume of Distribution
The volume of distribution of DPTA is 17 L.
Clearance
Pentetic acid presents a very rapid blood clearance which explains for the short half-life. The reported clearance rate in patients with normal renal function is 80-120ml/min.
Pentetic acid and its derivatives present a very minimal metabolism in the body.
In preclinical studies, DTPA has been shown to present a very short half-life of 18.5-31.8 min after intravenous administration.
The calcium and zinc trisodium salts of DTPA achieve the therapeutical potential by exchanging calcium and zinc cations with transuranic radionuclides to form higher affinity complexes and then promote their elimination by glomerular filtration into the urine. DTPA as an acid acts in a very similar way by sequestering ions with its eight coordinate bond forming sites.
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
50
PharmaCompass offers a list of Pentetic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pentetic Acid manufacturer or Pentetic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pentetic Acid manufacturer or Pentetic Acid supplier.
PharmaCompass also assists you with knowing the Pentetic Acid API Price utilized in the formulation of products. Pentetic Acid API Price is not always fixed or binding as the Pentetic Acid Price is obtained through a variety of data sources. The Pentetic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pentetic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pentetic Acid, including repackagers and relabelers. The FDA regulates Pentetic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pentetic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pentetic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pentetic Acid supplier is an individual or a company that provides Pentetic Acid active pharmaceutical ingredient (API) or Pentetic Acid finished formulations upon request. The Pentetic Acid suppliers may include Pentetic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Pentetic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pentetic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Pentetic Acid active pharmaceutical ingredient (API) in detail. Different forms of Pentetic Acid DMFs exist exist since differing nations have different regulations, such as Pentetic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pentetic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Pentetic Acid USDMF includes data on Pentetic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pentetic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pentetic Acid suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Pentetic Acid Drug Master File in Korea (Pentetic Acid KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Pentetic Acid. The MFDS reviews the Pentetic Acid KDMF as part of the drug registration process and uses the information provided in the Pentetic Acid KDMF to evaluate the safety and efficacy of the drug.
After submitting a Pentetic Acid KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Pentetic Acid API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Pentetic Acid suppliers with KDMF on PharmaCompass.
A Pentetic Acid written confirmation (Pentetic Acid WC) is an official document issued by a regulatory agency to a Pentetic Acid manufacturer, verifying that the manufacturing facility of a Pentetic Acid active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pentetic Acid APIs or Pentetic Acid finished pharmaceutical products to another nation, regulatory agencies frequently require a Pentetic Acid WC (written confirmation) as part of the regulatory process.
click here to find a list of Pentetic Acid suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pentetic Acid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pentetic Acid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pentetic Acid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pentetic Acid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pentetic Acid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pentetic Acid suppliers with NDC on PharmaCompass.
Pentetic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pentetic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pentetic Acid GMP manufacturer or Pentetic Acid GMP API supplier for your needs.
A Pentetic Acid CoA (Certificate of Analysis) is a formal document that attests to Pentetic Acid's compliance with Pentetic Acid specifications and serves as a tool for batch-level quality control.
Pentetic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Pentetic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pentetic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Pentetic Acid EP), Pentetic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pentetic Acid USP).

