
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
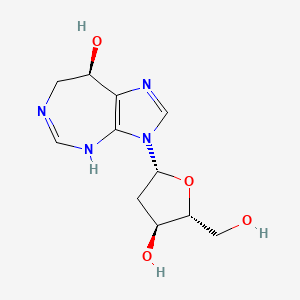

1. 2' Deoxycoformycin
2. 2'-deoxycoformycin
3. Ci 825
4. Ci-825
5. Ci825
6. Co-vidarabine
7. Deoxycoformycin
8. Imidazo(4,5-d)(1,3)diazepin-8-ol, 3-(2-deoxy-beta-d-erythro-pentofuranosyl)-3,4,7,8-tetrahydro-, (r)-
9. Nipent
10. Nsc 218321
11. Nsc-218321
12. Nsc218321
1. Deoxycoformycin
2. 53910-25-1
3. Nipent
4. 2'-deoxycoformycin
5. Covidarabine
6. 2'-dcf
7. Co-vidarabine
8. Ci-825
9. Vidarbine
10. Nsc-218321
11. Yk-176
12. Co-v
13. Pd 81565
14. Pd-81565
15. Pd-adi
16. 2'-dexoycoformycin
17. Vira A Deaminase Inhibitor
18. 395575mzo7
19. (8r)-3-(2-deoxy-beta-d-erythro-pentofuranosyl)-3,4,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
20. (8r)-3-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3h,6h,7h,8h-imidazo[4,5-d][1,3]diazepin-8-ol
21. Ncgc00182045-01
22. Deaminase Inhibitor (pd)
23. Nsc 218321
24. (8r)-3-(2-deoxy-beta-d-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
25. (r)-3-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3,4,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
26. (r)-3-(2-deoxy-.beta.-d-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo(4,5-d)(1,3)diazepin-8-ol
27. Pentostatina
28. Pentostatine
29. Pentostatinum
30. (r)-2'-deoxycoformycin
31. (r)-3-(2-deoxy-beta-d-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo(4,5-d)(1,3)diazepin-8-ol
32. (r)-deoxycoformycin
33. Cl 67310465
34. 8r-2'-deoxycoformycin
35. Pentostatine [inn-french]
36. Pentostatinum [inn-latin]
37. Pentostatina [inn-spanish]
38. Pentostatn
39. Pentostatin (jan/usan/inn)
40. Unii-395575mzo7
41. Hsdb 6547
42. (r)-3-(2-deoxy-beta-d-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
43. Pentostatin [usan:inn:ban:jan]
44. Pentostatin (ptn)
45. Brn 1223097
46. Pentostatin [mi]
47. Pentostatin [inn]
48. Pentostatin [jan]
49. Dsstox_cid_3436
50. Cl-67310465
51. Deaminase, Inhibitor For Adenosine Arabinoside
52. Pentostatin [hsdb]
53. Pentostatin [usan]
54. Pentostatin [vandf]
55. Pentostatin(deoxycoformycin)
56. Schembl2817
57. Chembl1580
58. Dsstox_rid_77027
59. Pentostatin [mart.]
60. Dsstox_gsid_23436
61. Pentostatin [who-dd]
62. Bidd:gt0136
63. Gtpl4805
64. Dtxsid2023436
65. Bdbm22925
66. Pentostatin, >=95% (hplc)
67. Pentostatin [orange Book]
68. Bdbm223291
69. (r)-3-((2s,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
70. Amy10324
71. Hy-a0006
72. Imidazo(4,5-d)(1,3)diazepin-8-ol, 3-(2-deoxy-.beta.-d-erythro-pentofuranosyl)-3,6,7,8-tetrahydro-, (r)-
73. Zinc3806262
74. Tox21_113417
75. Mfcd00078802
76. S9521
77. Akos024456918
78. Akos032949742
79. Cs-0374
80. Db00552
81. Ncgc00388420-02
82. Imidazo[4,5-d][1,3]diazepin-8-ol, 3-(2-deoxy-.beta.-d-erythro-pentofuranosyl)-3,4,7,8-tetrahydro-, (8r)-
83. Cas-53910-25-1
84. C02267
85. D00155
86. 910p251
87. A829822
88. Q425470
89. Sr-01000883935
90. J-523899
91. Sr-01000883935-1
92. Adenosine Deaminase Inhibitor, Dcf - Cas 53910-25-1
93. Z2216208604
94. (8r)-3-(2-deoxy-?-d-erythro-pentofuranosyl)-3,4,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
95. (8r)-3-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-3h,4h,7h,8h-imidazo[4,5-d][1,3]diazepin-8-ol
96. (8r)-3-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-7,8-dihydro-6h-imidazo[4,5-d][1,3]diazepin-8-ol
97. (r,z)-3-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol
98. Imidazo(4,5-d)(1,3)diazepin-8-ol, 3-(2-deoxy-beta-d-erythro-pentofuranosyl)-3,6,7,8-tetrahydro-, (r)-
| Molecular Weight | 268.27 g/mol |
|---|---|
| Molecular Formula | C11H16N4O4 |
| XLogP3 | -2.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 268.11715500 g/mol |
| Monoisotopic Mass | 268.11715500 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 356 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Nipent |
| PubMed Health | Pentostatin (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | NIPENT (pentostatin for injection) is supplied as a sterile, apyrogenic, lyophilized powder in single-dose vials for intravenous administration. Each vial contains 10 mg of pentostatin and 50 mg of Mannitol, USP. The pH of the final product is main... |
| Active Ingredient | Pentostatin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/vial |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 4 | |
|---|---|
| Drug Name | Pentostatin |
| PubMed Health | Pentostatin (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | NIPENT (pentostatin for injection) is supplied as a sterile, apyrogenic, lyophilized powder in single-dose vials for intravenous administration. Each vial contains 10 mg of pentostatin and 50 mg of Mannitol, USP. The pH of the final product is main... |
| Active Ingredient | Pentostatin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/vial |
| Market Status | Prescription |
| Company | Mylan Institutional; Eurohlth Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Nipent |
| PubMed Health | Pentostatin (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | NIPENT (pentostatin for injection) is supplied as a sterile, apyrogenic, lyophilized powder in single-dose vials for intravenous administration. Each vial contains 10 mg of pentostatin and 50 mg of Mannitol, USP. The pH of the final product is main... |
| Active Ingredient | Pentostatin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/vial |
| Market Status | Prescription |
| Company | Hospira |
| 4 of 4 | |
|---|---|
| Drug Name | Pentostatin |
| PubMed Health | Pentostatin (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | NIPENT (pentostatin for injection) is supplied as a sterile, apyrogenic, lyophilized powder in single-dose vials for intravenous administration. Each vial contains 10 mg of pentostatin and 50 mg of Mannitol, USP. The pH of the final product is main... |
| Active Ingredient | Pentostatin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/vial |
| Market Status | Prescription |
| Company | Mylan Institutional; Eurohlth Intl |
Antibiotics; Antineoplastic Agents; Enzyme Inhibitors; Immunosuppressive Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2000)
Antibiotics, Antineoplastic; Enzyme Inhibitors; Immunosuppressive Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Antineoplastic
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1131
Pentostatin is used for the palliative treatment of hairy cell leukemia (leukemic reticuloendotheliosis) that responds inadequately to, or progresses during, interferon alfa therapy. Pentostatin has been designated an orphan drug by the US Food and Drug Administration (FDA) for the treatment of this condition. ... Pentostatin produces clinically important tumor regression or disease stabilization (complete or partial responses) in approximately 80-100% of patients with hairy cell leukemia, including in previously untreated patients (eg, those who have not undergone splenectomy or other therapy) as well as in those in whom splenectomy and/or therapy with other agents (eg, interferons, antineoplastic agents) have failed to control the disease (eg, those with progressive disease). In clinical studies in patients with interferon alfa-refractory hairy cell leukemia, a complete response to pentostatin therapy generally was defined as clearing of peripheral blood and bone marrow of hairy cells; normalization of organomegaly and lymphadenopathy; and recovery of hemoglobin concentration to at least 12 g/dl, platelet count to at least 100,000/cu mm, and granulocyte count to at least 1500/cu mm. A partial response was defined as a decrease of greater than 50% in the number of hairy cells in peripheral blood and bone marrow and a decrease of greater than 50% in organomegaly and lymphadenopathy; hematologic parameters for a partial response were the same as those for a complete response. Overall complete and partial responses of 58 and 28%, respectively, reportedly were observed in a limited number of these patients receiving pentostatin 4 mg/sq m iv every other week for 3 mo; responding patients continued treatment for another 3-9 mos. The median time to response in these patients reportedly was 4.7 mo (range: 2.9-24.1 mo). The median duration of response to pentostatin therapy in 2 clinical studies of patients with hairy cell leukemia reportedly exceeded 7.7 and 15.2 mo, with relapse occurring in approximately 15-20% of patients showing an initial response. For patients with progressive, postsplenectomy disease, pentostatin generally has been considered an alternative to interferon alfa or secondary therapy for interferon refractory disease since experience with interferon alfa has been more extensive to date. However, superiority of either drug or of other therapies remains to be established.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
For more Therapeutic Uses (Complete) data for PENTOSTATIN (10 total), please visit the HSDB record page.
Pentostatin is a toxic drug with a low therapeutic index, and a therapeutic response is not likely to occur without some evidence of toxicity. The drug must be used only under constant supervision by physicians experienced in therapy with cytotoxic agents. Most, but not all, adverse effects of pentostatin are reversible if detected promptly. When severe adverse effects occur during pentostatin therapy, the drug should be discontinued or dosage reduced and appropriate measures instituted. Pentostatin should be reinstituted with caution if at all, with adequate consideration of further need for the drug, and with awareness of possible recurrence of toxicity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 636
Patients with poor performance status appear to experience greater toxicity with pentostatin and should be treated with the drug only when the anticipated benefits outweigh the potential risks.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 636
Hematologic function must be frequently and carefully monitored during and after pentostatin therapy, particularly during the first several courses of therapy in patients at increased risk of myelosuppression (eg, those with hairy cell leukemia). Initiation of pentostatin therapy in such patients can result in severe myelosuppression. If severe neutropenia continues beyond the initial cycles of pentostatin therapy, patients should be examined, including bone marrow examination, to determine the status of their disease. In addition, periodic monitoring for evidence of peripheral hairy cells should be performed in patients with this leukemia to evaluate the patient's response to therapy. Bone marrow aspirations and biopsies also may be required at 2 to 3 mo intervals.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 636
Patients receiving pentostatin should be observed closely for signs of nonhematologic (eg, neurologic) toxicity. If severe adverse reactions occur, the drug should be withheld and appropriate corrective measures taken as indicated. Therapy with pentostatin should be temporarily withheld or discontinued in patients who develop evidence of neurologic toxicity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 636
For more Drug Warnings (Complete) data for PENTOSTATIN (11 total), please visit the HSDB record page.
For the treatment of hairy cell leukaemia refractory to alpha interferon.
Pentostatin is an antineoplastic anti-metabolite used in the treatment of several forms of leukemia including acute nonlymphocytic leukemia and hairy cell leukemia. Anti-metabolites masquerade as purine or pyrimidine - which become the building blocks of DNA. They prevent these substances becoming incorporated in to DNA during the "S" phase (of the cell cycle), stopping normal development and division. It is a 6-thiopurine analogue of the naturally occurring purine bases hypoxanthine and guanine. Intracellular activation results in incorporation into DNA as a false purine base. An additional cytotoxic effect is related to its incorporation into RNA. Cytotoxicity is cell cycle phase-specific (S-phase).
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Adenosine Deaminase Inhibitors
Drugs that inhibit ADENOSINE DEAMINASE activity. (See all compounds classified as Adenosine Deaminase Inhibitors.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX08 - Pentostatin
Absorption
Not absorbed orally, crosses blood brain barrier.
Route of Elimination
In man, following a single dose of 4 mg/m2 of pentostatin infused over 5 minutes, approximately 90% of the dose was excreted in the urine as unchanged pentostatin and/or metabolites as measured by adenosine deaminase inhibitory activity.
Clearance
68 mL/min/m2
Plasma concentrations of pentostatin following direct iv injection of 0.25 mg/kg daily for 4 or 5 days in a limited number of patients with advanced, refractory cancer ranged from approximately 3.2-9.7 ng/ml. Plasma concentrations appear to increase linearly with dose; in a study in patients with leukemia, plasma pentostatin concentrations determined 1 hour after administration of 0.25 or 1 mg/kg of the drug as a 30 min iv infusion averaged approximately 0.4 or 1.26 ug/ml, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
No apparent correlation has been documented between mean or absolute plasma adenosine or deoxyadenosine concentrations and therapeutic or toxic responses to pentostatin; however, limited data suggest that there may be a correlation between response to the drug and the ratio of deoxyadenosine triphosphate to adenosine triphosphate in lymphoblasts. In addition, increases in plasma deoxyadenosine reportedly parallel the accumulation of deoxyadenosine triphosphate in erythrocytes and lymphoblasts, and there appears to be a correlation between toxicity and the ratio of deoxyadenosine triphosphate to adenosine triphosphate in erythrocytes.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
Studies in animals indicate that pentostatin distributes rapidly to all body tissues, but the extent of drug accumulation in different tissues appears to vary among species. Following intraperitoneal injection in mice, the highest concentrations of the drug were found in the kidneys, liver, and spleen. In dogs, pentostatin tissue concentrations following iv administration were proportional to tissue adenosine deaminase activity, with the highest concentrations in the lungs, spleen, pancreas, heart, liver, and jejunum. Pentostatin reportedly enters erythrocytes via a facilitated transport system common to other nucleosides or by simple diffusion; efflux of the drug from cells has not been characterized, although the time course of pentostatin's effects (eg, adenosine deaminase inhibition) varies among different types of cells (eg, lymphocytes, erythrocytes).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
Limited data in animals and humans indicate that pentostatin distributes relatively poorly into CSF, with peak CSF concentrations averaging approximately 10% of concurrent plasma concentrations. In a 6 yr old leukemia patient receiving pentostatin 0.25 mg/kg daily for 3 successive days by direct iv injection, serum and CSF (via lumbar puncture) pentostatin concentrations 4 hr after the initial dose were approximately 147 and 19 ng/ml, respectively, using an enzyme-inhibition titration assay; one hour after the third dose, corresponding serum and CSF concentrations were approximately 241 and 35 ng/ml, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
For more Absorption, Distribution and Excretion (Complete) data for PENTOSTATIN (7 total), please visit the HSDB record page.
Primarily hepatic, but only small amounts are metabolized.
5.7 hours (with a range between 2.6 and 16 hrs)
Following iv administration of 4 mg/sq m of pentostatin as a single dose over 5 min in healthy individuals, the distribution half-life and terminal elimination half-life reportedly averaged 11 min and 5.7 hr, respectively. In a multiple dose study in a limited number of patients receiving 36 courses of pentostatin at a dosage of 4 mg/sq m iv, distribution half-life and terminal elimination half-life reportedly averaged 9.6 min (range: 3.1-48.5 min) and 4.9 hr, respectively. In other studies in a limited number of patients with advanced cancer, the distribution half-life averaged 17-85 min and the terminal elimination half-life averaged 2.6-15 hr following single iv doses of 0.1 or 0.25 mg/kg of pentostatin.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
In patients with renal impairment (creatinine clearance less than 60 ml/min),the half-life of pentostatin averages approximately 18 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
Pentostatin is a potent transition state inhibitor of adenosine deaminase (ADA), the greatest activity of which is found in cells of the lymphoid system. T-cells have higher ADA activity than B-cells, and T-cell malignancies have higher activity than B-cell malignancies. The cytotoxicity that results from prevention of catabolism of adenosine or deoxyadenosine is thought to be due to elevated intracellular levels of dATP, which can block DNA synthesis through inhibition of ribonucleotide reductase. Intracellular activation results in incorporation into DNA as a false purine base. An additional cytotoxic effect is related to its incorporation into RNA. Cytotoxicity is cell cycle phase-specific (S-phase).
... Adenosine deaminase inhibitor
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1131
The precise mechanism(s) of action of pentostatin in hairy cell leukemia and other lymphoid malignancies has not been fully elucidated. Pentostatin is a potent transition state (tight binding) inhibitor of adenosine deaminase, an enzyme involved in purine metabolism. This enzyme appears to regulate intracellular adenosine concentrations via irreversible deamination of adenosine and deoxyadenosine. Although adenosine deaminase is widely distributed in mammalian tissues, highest levels are found in lymphoid tissue: levels in circulating T cells (particularly in T cell lymphoblastic leukemia) are higher than those in B cells. While the level of enzyme activity is low in healthy bone marrow, it is high in myeloid leukemic blast cells. ... Inhibition of adenosine deaminase by pentostatin results in intracellular accumulation of toxic levels of adenine deoxynucleotides (eg, deoxyadenosine triphosphate), which in the presence of deoxyadenosine can lead to cell death. Pentostatin alone, even in concentrations high enough to inhibit adenosine deaminase completely, is not cytotoxic to lymphoid cells cultured in the absence of cytotoxic nucleosides (eg, deoxyadenosine). Thus, unlike many other nucleoside-analog antineoplastic agents, the cytoxic effects of pentostatin do not appear to be attributable directly to the drug or its metabolites but instead appear to result indirectly from the effects of the substrates for adenosine deaminase (adenosine and deoxyadenosine) and/or their metabolites. Although elevated deoxyadenosine triphosphate concentrations in the cell can block DNA synthesis via inhibition of ribonucleotide reductase, the precise role of high deoxyadenosine triphosphate concentrations in pentostatin-induced cytotoxicity is controversial. Pentostatin also can inhibit RNA synthesis, cause DNA strand breaks, disrupt ATP-dependent cellular processes, and inhibit adenosylhomocysteinase (S-adenosylhomocysteine hydrolase), all of which also may contribute to the drug's lymphocytotoxic effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
The degree to which pentostatin inhibits adenosine deaminase varies among cell types, possibly because of differences in enzyme inhibitor dissociation constants in different cells as well as differences in cellular accumulation of the drug. There generally has been no clear relation between adenosine deaminase inhibition and pentostatin induced cytotoxicity in clinical studies. However, the cytotoxic and growth inhibitory effects of adenosine deaminase inhibition appear to be greater in T cells than in B cells. Although conflicting data exist, some evidence suggests that T cells accumulate more deoxyadenosine triphosphate than B cells and thus may be more susceptible to the effects of adenosine deaminase inhibition; deoxyadenosine triphosphate concentrations in B cells may be lower because these cells possess higher membrane associated ecto-5'-nucleotidase activity, which promotes the hydrolysis of higher phosphate compounds to more freely diffusible nucleosides. Differences in the sensitivity of B and T cells to pentostatin's effects also may be artifactual as a result of testing procedure variables (eg, cell source, culture media conditions). The time course of adenosine deaminase inhibition appears to differ in erythrocytes and lymphocytes and depends on the intrinsic activity of the enzyme in the cell as well as cell specific pharmacodynamics (eg, protein synthesis, rate of cellular proliferation). In some cells, inhibition by a single dose of pentostatin may persist for 1 week or longer. It is not known whether recovery from adenosine deaminase inhibition occurs as a result of slow efflux of pentostatin from the cell or regeneration of adenosine deaminase; however, recovery of blood adenosine deaminase activity may result from replenishment of enzyme from newly formed erythrocytes in that such recovery in animals has been reported to coincide with the life span of erythrocytes in circulation (eg, 40-60 days). ... Response to pentostatin varies according to the type and sensitivity of the neoplasm being treated. Conditions associated with relatively low adenosine deaminase activity (eg, hairy cell and chronic lymphocytic leukemias) manifest prolonged and complete adenosine deaminase inhibition in response to relatively low dosages of pentostatin, whereas conditions associated with high adenosine deaminase activity (eg, acute leukemias) are less sensitive to the drug, requiring higher doses that produce relatively incomplete inhibition of adenosine deaminase activity.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 633
Related Excipient Companies

Excipients by Applications
Global Sales Information

ABOUT THIS PAGE
87
PharmaCompass offers a list of Pentostatin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pentostatin manufacturer or Pentostatin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pentostatin manufacturer or Pentostatin supplier.
PharmaCompass also assists you with knowing the Pentostatin API Price utilized in the formulation of products. Pentostatin API Price is not always fixed or binding as the Pentostatin Price is obtained through a variety of data sources. The Pentostatin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pentostatin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pentostatin, including repackagers and relabelers. The FDA regulates Pentostatin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pentostatin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pentostatin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pentostatin supplier is an individual or a company that provides Pentostatin active pharmaceutical ingredient (API) or Pentostatin finished formulations upon request. The Pentostatin suppliers may include Pentostatin API manufacturers, exporters, distributors and traders.
click here to find a list of Pentostatin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pentostatin DMF (Drug Master File) is a document detailing the whole manufacturing process of Pentostatin active pharmaceutical ingredient (API) in detail. Different forms of Pentostatin DMFs exist exist since differing nations have different regulations, such as Pentostatin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pentostatin DMF submitted to regulatory agencies in the US is known as a USDMF. Pentostatin USDMF includes data on Pentostatin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pentostatin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pentostatin suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pentostatin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pentostatin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pentostatin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pentostatin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pentostatin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pentostatin suppliers with NDC on PharmaCompass.
Pentostatin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pentostatin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pentostatin GMP manufacturer or Pentostatin GMP API supplier for your needs.
A Pentostatin CoA (Certificate of Analysis) is a formal document that attests to Pentostatin's compliance with Pentostatin specifications and serves as a tool for batch-level quality control.
Pentostatin CoA mostly includes findings from lab analyses of a specific batch. For each Pentostatin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pentostatin may be tested according to a variety of international standards, such as European Pharmacopoeia (Pentostatin EP), Pentostatin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pentostatin USP).
