
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
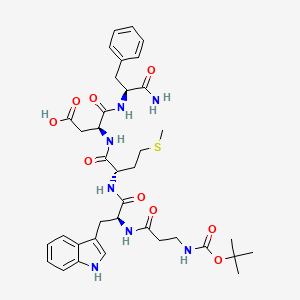

1. Acignost
2. Gastrin Pentapeptide
3. Pentapeptide, Gastrin
4. Peptavlon
1. Peptavlon
2. 5534-95-2
3. Ici-50123
4. Ay-6608
5. Ici 50,123
6. Chembl1328
7. Ef0nx91490
8. Nsc-367746
9. Dsstox_cid_28918
10. Dsstox_rid_83185
11. Dsstox_gsid_48992
12. Gastrodiagnost
13. Pentagastrina
14. Pentagastrine
15. Pentagastrinum
16. Petogasrin
17. Cas-5534-95-2
18. Ici 50123
19. Pentagastrine [inn-french]
20. Pentagastrinum [inn-latin]
21. Pentagastrina [inn-spanish]
22. Unii-ef0nx91490
23. Hsdb 3247
24. Peptavlon (tn)
25. Boc-beta-ala-try-met-asp-phe(nh2)
26. Einecs 226-889-7
27. Pentagastrin [usan:inn:ban:jan]
28. Mfcd00076515
29. Nsc 367746
30. Ay 6608
31. Brn 5472892
32. Pentagastrin [mi]
33. Pentagastrin [inn]
34. Pentagastrin [jan]
35. Pentagastrin [hsdb]
36. Pentagastrin [usan]
37. Pentagastrin [vandf]
38. Schembl26045
39. Pentagastrin [mart.]
40. L-phenylalaninamide, N-((1,1-dimethylethoxy)carbonyl)-beta-alanyl-l-tryptophyl-l-methionyl-l-alpha-aspartyl-
41. Mls006009992
42. Pentagastrin [who-dd]
43. Pentagastrin (jan/usan/inn)
44. Dtxsid3048992
45. Chebi:31974
46. Pentagastrin [orange Book]
47. (3s)-4-[[(2s)-1-amino-1-oxo-3-phenylpropan-2-yl]amino]-3-[[(2s)-2-[[(2s)-3-(1h-indol-3-yl)-2-[3-[(2-methylpropan-2-yl)oxycarbonylamino]propanoylamino]propanoyl]amino]-4-methylsulfanylbutanoyl]amino]-4-oxobutanoic Acid
48. Hy-a0261
49. Zinc8214644
50. Boc-
51. A-ala-trp-met-asp-phe-nh2
52. Boc-beta-ala-trp-met-asp-phe-nh2
53. Tox21_113479
54. Bdbm50024321
55. Akos030213248
56. Tox21_113479_1
57. Cs-5667
58. Db00183
59. N-t-butyloxycarbonyl-beta-alanyl-l-tryptophyl-l-methion Yl-l-aspartyl-l-phenylalanine Amide
60. Ncgc00167300-01
61. Ncgc00183362-01
62. Ncgc00183362-02
63. Alaninamide, N-carboxy-beta-alanyl-l-tryptophyl-l-methionyl-l-aspartylphenyl-, N-tert-butyl Ester, L-
64. As-56396
65. N-(alpha-carbamoylphenethyl)-3-(2-(2-(3-(carboxyamino)propionamido)-3-indol-3-ylpropionamido)-4-(methylthio)butyramido)succinamic Acid N-tert-butyl Ester
66. N-(n-(n-(n-(n-tert-butoxycarbonyl-beta-alanyl)-l-tryptophanyl)-l-methionyl)-l-aspartyl)-l-phenylalaninamide
67. N-carboxy-beta-alanyl-l-tryptophyl-l-methionyl-l-aspartylphenyl-l-alaninamide N-tert-butyl Ester
68. Smr004701067
69. D01631
70. 534p952
71. Q423586
72. (10s,13s,16s)-10-((1h-indol-3-yl)methyl)-16-((s)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)-2,2-dimethyl-13-(2-(methylthio)ethyl)-4,8,11,14-tetraoxo-3-oxa-5,9,12,15-tetraazaoctadecan-18-oic Acid
73. 3-{2-[2-(3-tert-butoxycarbonylamino-propionylamino)-3-(1h-indol-3-yl)-propionylamino]-4-methylsulfanyl-butyrylamino}-n-(1-carbamoyl-2-phenyl-ethyl)-succinamic Acid
74. Alaninamide, N-carboxy-beta-alanyl-l-tryptophyl-l-methionyl-l-aspartylphenyl-, N-tert-butylester, L-
75. L-phenylalaninamide, N-((1,1-dimethylethoxy)carbonyl)-.beta.-alanyl-l-tryptophyl-l-methionyl-l-.alpha.-aspartyl-
76. N-carboxy-.beta.-alanyl-l-tryptophyl-l-methionyl-l-aspartylphenyl-l-alaninamide N-tert-butyl Ester
| Molecular Weight | 767.9 g/mol |
|---|---|
| Molecular Formula | C37H49N7O9S |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 22 |
| Exact Mass | 767.33124734 g/mol |
| Monoisotopic Mass | 767.33124734 g/mol |
| Topological Polar Surface Area | 276 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 1310 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
PENTAGASTRIN...USED WIDELY IN EUROPE SINCE 1966 TO MEASURE MAXIMAL ACID SECRETORY CAPACITY OF STOMACH. IT ELICITS REPRODUCIBLE SECRETORY RESPONSES COMPARABLE TO THOSE INDUCED BY HISTAMINE OR BETAZOLE...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
.../IT/ OFFERS SEVERAL ADVANTAGES /TO HISTAMINE & BETAZOLE/. ...REQUIRES ONLY SINGLE SC OR IM INJECTION; IT IS RELATIVELY SHORT IN DURATION...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
PENTAGASTRIN HAS DISTINCT EFFECT ON RATIOS OF NA+, K+, H+/AMINO ACID IN GASTRIC JUICE IN DIFFERENT STATES OF ACIDITY, VALUES OF ALL QUOTIENTS IN PT WITH HYPER, HYPO & ANACIDITY ARE SIGNIFICANTLY REDUCED.
DUNZENDORF R U; ARZNEIM FORSCH 26 (7) 1364-1365 (1976)
Used as a diagnostic aid for evaluation of gastric acid secretory function
Pentagastrin is indicated as a diagnostic aid for evaluation of gastric acid secretory function. It is effective in testing for anacidity (achlorhydria) in patients with suspected pernicious anemia, atrophic gastritis, or gastric carcinoma. It is also effective in determining the reduction in acid output after operations for peptic ulcer, such as vagotomy or gastric resection.
V - Various
V04 - Diagnostic agents
V04C - Other diagnostic agents
V04CG - Tests for gastric secretion
V04CG04 - Pentagastrin
Absorption
Rapidly absorbed after parenteral administration.
ACTIVITY OF ORAL PENTAGASTRIN WAS DETERMINED BY ITS ACTION AS GASTRIC ACID STIMULANT IN 4 HEALTHY SUBJECTS, 2 PT WITH DUODENAL ULCERS, & 1 PT WITH ANNULAR PANCREAS IN ASSOC WITH DUODENAL ULCER.
MORRELL MT, KEYNES WM; ABSORPTION OF PENTAGASTRIN FROM GASTROINTESTINAL TRACT IN MAN; LANCET 2 (OCT 11) 712 (1975)
Primarily hepatic
10 minutes or less
HALF-LIFE OF PENTAGASTRIN IN CIRCULATION APPEARS TO BE ABOUT 10 MIN...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
The exact mechanism by which pentagastrin stimulates gastric acid, pepsin, and intrinsic factor secretion is unknown; however, since pentagastrin is an analogue of natural gastrin, it is believed that it excites the oxyntic cells of the stomach to secrete to their maximum capacity. Pentagastrin stimulates pancreatic secretion, especially when administered in large intramuscular doses. Pentagastrin also increases gastrointestinal motility by a direct effect on the intestinal smooth muscle. However, it delays gastric emptying time probably by stimulation of terminal antral contractions, which enhance retropulsion.
MOST PROMINENT ACTION OF PENTAGASTRIN IS TO STIMULATE SECRETION OF GASTRIC ACID, PEPSIN, & INTRINSIC FACTOR OF CASTLE...STIMULATES PANCREATIC SECRETION, INHIBITS ABSORPTION OF WATER & ELECTROLYTES FROM ILEUM, CONTRACTS SMOOTH MUSCLE OF LOWER ESOPHAGEAL SPHINCTER & STOMACH (BUT DELAYS GASTRIC EMPTYING TIME)...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
.../IT/ RELAXES SPHINCTER OF ODDI, INCR BLOOD FLOW IN GASTRIC MUCOSE, STIMULATES L-HISTIDINE DECARBOXYLASE ACTIVITY IN RAT GASTRIC MUCOSA, &, IN HIGH DOSES, STIMULATES VARIETY OF SMOOTH MUSCLES IN DIFFERENT SPECIES.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
IT ALSO MIMICS OR BLOCKS EFFECTS OF POLYPEPTIDES PANCREOZYMIN-CHOLECYSTOKININ, SECRETIN, CAERULIN, NATURALLY OCCURRING DECAPEPTIDE THAT, ALONG WITH PANCREOZYMIN-CHOLECYSTOKININ, SHARES COMMON C-TERMINAL HEPTAPEPTIDE RESIDUE WITH GASTRIN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
PENTAGASTRIN ACTIVATES ADENYLATE CYCLASE IN GASTRIC MUCOSA...
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 601
PENTAGASTRIN INITIALLY PRODUCED MARKED ANTRAL ACTIVITY IN PHYSIOLOGICAL STRICTURE & SUBSEQUENT DELAY IN OVERALL RATE OF GASTRIC EMPTYING. FUNDAL MOTILITY WAS UNAFFECTED THOUGH REFLUX FROM ANTRUM OCCURRED.
HAMILTON SG ET AL; GUT 17 (4) 273-279 (1976)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
97
PharmaCompass offers a list of Peptavlon API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Peptavlon manufacturer or Peptavlon supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Peptavlon manufacturer or Peptavlon supplier.
PharmaCompass also assists you with knowing the Peptavlon API Price utilized in the formulation of products. Peptavlon API Price is not always fixed or binding as the Peptavlon Price is obtained through a variety of data sources. The Peptavlon Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Peptavlon manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Peptavlon, including repackagers and relabelers. The FDA regulates Peptavlon manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Peptavlon API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Peptavlon manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Peptavlon supplier is an individual or a company that provides Peptavlon active pharmaceutical ingredient (API) or Peptavlon finished formulations upon request. The Peptavlon suppliers may include Peptavlon API manufacturers, exporters, distributors and traders.
click here to find a list of Peptavlon suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Peptavlon Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Peptavlon GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Peptavlon GMP manufacturer or Peptavlon GMP API supplier for your needs.
A Peptavlon CoA (Certificate of Analysis) is a formal document that attests to Peptavlon's compliance with Peptavlon specifications and serves as a tool for batch-level quality control.
Peptavlon CoA mostly includes findings from lab analyses of a specific batch. For each Peptavlon CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Peptavlon may be tested according to a variety of international standards, such as European Pharmacopoeia (Peptavlon EP), Peptavlon JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Peptavlon USP).
