
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
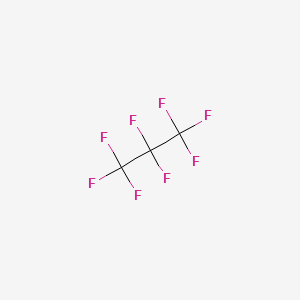

1. C3f8-gas
2. Definity
3. Mrx-115
4. Octafluoropropane
5. Perfluoropropane
1. Perfluoropropane
2. Octafluoropropane
3. Definity
4. 76-19-7
5. Propane, Octafluoro-
6. 1,1,1,2,2,3,3,3-octafluoropropane
7. Freon 218
8. Optison
9. Oktafluorpropan
10. Genetron 218
11. Dmp 115
12. Mrx-115
13. Octafluorpropan
14. 9048-46-8
15. Fc 218
16. Fc 218 (refrigerant)
17. Bovine Serum Albumin
18. Fs069
19. Hfc 218
20. Pfc 218
21. Un 2424
22. Dmp-115
23. R 218
24. Fs-069
25. Ck0n3wh0sr
26. Luminity
27. Chebi:31980
28. Perflutren [usan]
29. Propane, 1,1,1,2,2,3,3,3-octafluoro-
30. C3f8-gas
31. Perflutren Lipid Microsphere
32. Einecs 200-941-9
33. Unii-ck0n3wh0sr
34. Un2424
35. Perflutren Lipid Microspheres
36. Bovine Albumin
37. Perflutren [usan:inn:ban]
38. C3f8
39. Hsdb 8074
40. Octafluoro-propane
41. Definity (tn)
42. Mfcd00039239
43. Optison (salt/mix)
44. Perflutren [ii]
45. Perflutren [inn]
46. Perflutren [jan]
47. Perflutren [vandf]
48. Ec 200-941-9
49. Perflutren-lipid Microsphere
50. Perflutren [mart.]
51. Perflutren Protein-type A Microspheres Injection
52. Chembl1663
53. Perflutren [who-dd]
54. Perfluoropropane [mi]
55. Perflutren (jan/usan/inn)
56. Perflutren [ema Epar]
57. Perfluoropropane [inci]
58. Dtxsid9052503
59. Perflutren [orange Book]
60. Octafluoropropane [vandf]
61. Oxtafluoropropane [vandf]
62. Amy6865
63. Mrx 115
64. Octafluoropropane-lipid Microsphere
65. Ym454
66. Zinc8214651
67. Akos006228213
68. Db00556
69. Fs-6566
70. Octafluoropropaneor Refrigerant Gas R 218
71. Db-056033
72. Ft-0621948
73. Ft-0631321
74. Liposome-encapsulated Perfluoropane Microsphere
75. 1,1,1,2,2,3,3,3-octakis(fluoranyl)propane
76. D01738
77. A838638
78. Q412659
79. Octafluoropropaneor Refrigerant Gas R 218 [un2424] [nonflammable Gas]
| Molecular Weight | 188.02 g/mol |
|---|---|
| Molecular Formula | C3F8 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 0 |
| Exact Mass | 187.98722530 g/mol |
| Monoisotopic Mass | 187.98722530 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Definity |
| PubMed Health | Perflutren Lipid Microsphere (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Active Ingredient | Perflutren |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 6.52mg/ml |
| Market Status | Prescription |
| Company | Lantheus Medcl |
| 2 of 4 | |
|---|---|
| Drug Name | Optison |
| PubMed Health | Perflutren Protein Type A Microsphere (Injection) |
| Drug Classes | Diagnostic Agent, Cardiac Function |
| Active Ingredient | Albumin human |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Definity |
| PubMed Health | Perflutren Lipid Microsphere (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Active Ingredient | Perflutren |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 6.52mg/ml |
| Market Status | Prescription |
| Company | Lantheus Medcl |
| 4 of 4 | |
|---|---|
| Drug Name | Optison |
| PubMed Health | Perflutren Protein Type A Microsphere (Injection) |
| Drug Classes | Diagnostic Agent, Cardiac Function |
| Active Ingredient | Albumin human |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Ge Healthcare |
Contrast Media; Fluorocarbons
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
THERAPEUTIC CATEGORY: Diagnostic aid (ultrasound contrast agent). Adjunct in repair of retinal detachment
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 2006., p. 1236
Activated Perflutren Lipid Microsphere Injectable Suspension is indicated for use in patients with suboptimal echocardiograms to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
/BOXED WARNING/ Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following perflutren-containing microsphere administration. Most serious reactions occur within 30 minutes of administration. Assess all patients for the presence of any condition that precludes perflutren-containing microsphere administration. Always have resuscitation equipment and trained personnel readily available.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
Do not administer Perflutren to patients with known or suspected: right-to-left, bi-directional, or transient right-to-left cardiac shunts; hpersensitivity to perflutren. Do not administer Perflutren by intra-arterial injection.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
Serious cardiopulmonary reactions including fatalities have occurred uncommonly during or shortly following perflutren-containing microsphere administration, typically within 30 minutes of administration. The risk for these reactions may be increased among patients with unstable cardiopulmonary conditions (acute myocardial infarction, acute coronary artery syndromes, worsening or unstable congestive heart failure, or serious ventricular arrhythmias). Always have cardiopulmonary resuscitation personnel and equipment readily available prior to Perflutren administration and monitor all patients for acute reactions. The reported reactions include: fatal cardiac or respiratory arrest, shock, syncope, symptomatic arrhythmias (atrial fibrillation, tachycardia, bradycardia, supraventricular tachycardia, ventricular fibrillation, ventricular tachycardia), hypertension, hypotension, dyspnea, hypoxia, chest pain, respiratory distress, stridor, wheezing, loss of consciousness, and convulsions.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
In postmarketing use, uncommon but serious anaphylactoid reactions were observed during or shortly following perflutren-containing microsphere administration including: Shock, hypersensitivity, bronchospasm, throat tightness, angioedema, edema (pharyngeal, palatal, mouth, peripheral, localized), swelling (face, eye, lip, tongue, upper airway), facial hypoesthesia, rash, urticaria, pruritus, flushing, and erythema have occurred in patients with no prior exposure to perflutren-containing microsphere products.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
For more Drug Warnings (Complete) data for Octafluoropropane (17 total), please visit the HSDB record page.
Used as an ultrasound contrast imaging in cardiology and radiology.
FDA Label
This medicinal product is for diagnostic use only.
Luminity is an ultrasound contrast-enhancing agent for use in patients in whom non-contrast echocardiography was suboptimal (suboptimal is considered to indicate that at least two of six segments in the 4- or 2-chamber view of the ventricular border were not evaluable) and who have suspected or established coronary artery disease, to provide opacification of cardiac chambers and improvement of left ventricular endocardial border delineation at both rest and stress.
This medicinal product is for diagnostic use only.
Optison is a transpulmonary echocardiographic contrast agent for use in patients with suspected or established cardiovascular disease to provide opacification of cardiac chambers, enhance left-ventricular-endocardial-border delineation with resulting improvement in wall-motion visualisation.
Optison should only be used in patients where the study without contrast enhancement is inconclusive.
Perflutren, a diagnostic drug that is intended to be used for contrast enhancement during the indicated echocardiographic procedures, comprised of lipid-coated microspheres filled with octafluoropropane(OFP) gas. It provide contrast enhancement of the endocardial borders during echocardiography. The perflutren lipid microspheres exhibit lower acoustic impedance than blood and enhance the intrinsic backscatter of blood.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08DA04
V08DA01
Human pharmacokinetics information is not available for the intact or degassed lipid microspheres. The pharmacokinetics of octafluoropropane gas (OFP) was evaluated in healthy subjects (n=8) after the IV administration of activated perflutren-containing microspheres at a 50 uL/kg dose. ... OFP was not detectable after 10 minutes in most subjects either in the blood or in expired air.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
OFP gas binding to plasma proteins or partitioning into blood cells has not been studied. However, OFP protein binding is expected to be minimal due to its low partition coefficient into whole blood.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
It is not known whether perflutren-containing microspheres are excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
A physiologically based pharmacokinetic model was developed to evaluate the kinetics of one of the newest sonographic contrast agents available, FS069 or Optison. This material consists of octafluoropropane gas encapsulated in proteinaceous microspheres, injected intravenously for use as a myocardial contrast agent in humans. This model has six compartments: two lung compartments (alveolar and dead volume), and compartments for the heart, slowly perfused tissue, richly perfused tissue, and gastrointestinal tract. The model was developed to determine the distribution and excretion of the octafluoropropane in the body. Despite the high affinity of octafluoropropane for tissue, the model predicted that nearly 100% of the material would be exhaled from the lungs within 6 min. The model verified the results of a phase I clinical trial with 10 healthy subjects. Ventilation rate was found to play a critical role in the complete excretion of this contrast agent. ...
PMID:9952073 Hutter JC et al; J Ultrasound Med 18 (1): 1-11 (1999)
OFP is not metabolized. The phospholipid components of the microspheres are thought to be metabolized to free fatty acids.
OFP is a stable gas that is not metabolized. The phospholipid components of the microspheres are thought to be metabolized to free fatty acids.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
The mean half-life of OFP in blood 1.9 minutes
OFP concentrations in blood were shown to decline in a mono-exponential fashion with a mean half-life of 1.3 minutes in healthy subjects.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
The pharmacokinetics of octafluoropropane gas (OFP) was evaluated in subjects (n=11) with chronic obstructive pulmonary disease (COPD). The mean half-life of OFP in blood was 1.9 minutes. The total lung clearance of OFP was similar to that in healthy subjects.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
Perflutren is comprised of gas-filled microspheres that are injected or infused into the body. When exposed to ultrasound waves, the microspheres resonate and "echo" strong signals back to the ultrasound machine. The difference in density between the gas-filled bubbles and the blood around them creates an increased level of contrast visible in the resulting ultrasound image. During echocardiography, activated Perflutren enhances images of the inner edges or borders of the heart, producing an improved image that may enable physicians to better diagnose patients.
Perflutren lipid microspheres exhibit lower acoustic impedance than blood and enhance the intrinsic backscatter of blood. These physical acoustic properties of activated perflutren-containing microspheres provide contrast enhancement of the left ventricular chamber and aid delineation of the left ventricular endocardial border during echocardiography.
US Natl Inst Health; DailyMed. Current Medication Information for DEFINITY (perflutren) injection, suspension (October 2011). Available from, as of June 1, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ab9c79c-1b5c-4e86-899c-cc74686f070a
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
68
PharmaCompass offers a list of Perflutren API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Perflutren manufacturer or Perflutren supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Perflutren manufacturer or Perflutren supplier.
PharmaCompass also assists you with knowing the Perflutren API Price utilized in the formulation of products. Perflutren API Price is not always fixed or binding as the Perflutren Price is obtained through a variety of data sources. The Perflutren Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Perflutren manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Perflutren, including repackagers and relabelers. The FDA regulates Perflutren manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Perflutren API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Perflutren manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Perflutren supplier is an individual or a company that provides Perflutren active pharmaceutical ingredient (API) or Perflutren finished formulations upon request. The Perflutren suppliers may include Perflutren API manufacturers, exporters, distributors and traders.
click here to find a list of Perflutren suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Perflutren DMF (Drug Master File) is a document detailing the whole manufacturing process of Perflutren active pharmaceutical ingredient (API) in detail. Different forms of Perflutren DMFs exist exist since differing nations have different regulations, such as Perflutren USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Perflutren DMF submitted to regulatory agencies in the US is known as a USDMF. Perflutren USDMF includes data on Perflutren's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Perflutren USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Perflutren suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Perflutren Drug Master File in Japan (Perflutren JDMF) empowers Perflutren API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Perflutren JDMF during the approval evaluation for pharmaceutical products. At the time of Perflutren JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Perflutren suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Perflutren Drug Master File in Korea (Perflutren KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Perflutren. The MFDS reviews the Perflutren KDMF as part of the drug registration process and uses the information provided in the Perflutren KDMF to evaluate the safety and efficacy of the drug.
After submitting a Perflutren KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Perflutren API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Perflutren suppliers with KDMF on PharmaCompass.
Perflutren Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Perflutren GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Perflutren GMP manufacturer or Perflutren GMP API supplier for your needs.
A Perflutren CoA (Certificate of Analysis) is a formal document that attests to Perflutren's compliance with Perflutren specifications and serves as a tool for batch-level quality control.
Perflutren CoA mostly includes findings from lab analyses of a specific batch. For each Perflutren CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Perflutren may be tested according to a variety of international standards, such as European Pharmacopoeia (Perflutren EP), Perflutren JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Perflutren USP).
