
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDA Orange Book

0
Europe

0
Canada

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
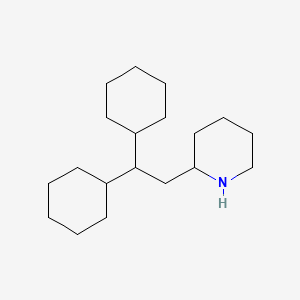

1. Perhexilene
1. Perhexilene
2. 2-(2,2-dicyclohexylethyl)piperidine
3. 6621-47-2
4. (+)-2-(2,2-dicyclohexylethyl)piperidine
5. Perhexilinum [inn-latin]
6. Perhexilina [inn-spanish]
7. Piperidine, 2-(2,2-dicyclohexylethyl)-
8. (-)-2-(2,2-dicyclohexylethyl)piperidine
9. 39648-48-1
10. Perhexiline (inn)
11. 39648-47-0
12. Chembl75880
13. Chebi:35553
14. Einecs 252-426-3
15. Ku65374x44
16. Perhexilline
17. Perhexilina
18. (1)-2-(2,2-dicyclohexylethyl)piperidine
19. Perhexiline [inn]
20. Perhexiline [inn:ban]
21. 10118-35-1
22. Einecs 229-569-5
23. Unii-ku65374x44
24. 2-[2,2-dicyclohexylethyl]piperidine Maleate Salt
25. Einecs 254-558-7
26. Einecs 254-559-2
27. Piperidine, 2-(2,2-dicyclohexylethyl)-, (2z)-2-butenedioate (1:1)
28. Spectrum_000013
29. Perhexiline [mi]
30. Prestwick0_000286
31. Prestwick1_000286
32. Prestwick2_000286
33. Prestwick3_000286
34. Spectrum2_001539
35. Spectrum3_001579
36. Spectrum4_000173
37. Spectrum5_001084
38. Oprea1_365504
39. Bspbio_000192
40. Bspbio_003118
41. Kbiogr_000685
42. Kbioss_000353
43. Perhexiline [who-dd]
44. Divk1c_000542
45. Schembl114894
46. Spbio_001358
47. Spbio_002411
48. Bpbio1_000212
49. Dtxsid7023439
50. Bdbm61402
51. Cid_5284439
52. Kbio1_000542
53. Kbio2_000353
54. Kbio2_002921
55. Kbio2_005489
56. Kbio3_002618
57. Ninds_000542
58. Hy-b1334
59. Db01074
60. Idi1_000542
61. Ncgc00018261-02
62. Ncgc00018261-05
63. 35193-73-8
64. (?)-2-(2,2-dicyclohexylethyl)piperidine
65. Sbi-0051794.p002
66. Ab00053656
67. Cs-0013087
68. D08340
69. 2-(2,2-dicyclohexylethyl)piperidine;maleic Acid
70. Ab00053656_22
71. (+/-)-2-(2,2-dicyclohexylethyl)piperidine
72. Q1232737
73. (z)-2-butenedioic Acid;2-(2,2-dicyclohexylethyl)piperidine
| Molecular Weight | 277.5 g/mol |
|---|---|
| Molecular Formula | C19H35N |
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 4 |
| Exact Mass | 277.276950121 g/mol |
| Monoisotopic Mass | 277.276950121 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 245 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the management of severe angina pectoris.
Used in the treatment of unresponsive or refractory angina. Perhexiline increases glucose metabolism at the expense of free-fatty-acid metabolism, enhancing oxygen efficiency during myocardial ischaemia. Perhexiline also potentiates platelet responsiveness to nitric oxide both in patients with angina and patients with acute coronary syndrome. The predominant mechanism of this particular perhexiline effect is an increase in platelet cGMP responsiveness. Perhexiline also may reduce the potential for nitric oxide clearance by neutrophil-derived oxygen. Perhexiline relieves symptoms of angina, improves exercise tolerance, and increases the workload needed to induce ischaemia when used as monotherapy. The primary therapeutic roles for perhexiline are as short-term therapy (less than 3 months duration) in patients with severe ischaemia awaiting coronary revascularisation or long-term therapy in patients with ischaemic symptoms refractory to other therapeutic measures.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C08 - Calcium channel blockers
C08E - Non-selective calcium channel blockers
C08EX - Other non-selective calcium channel blockers
C08EX02 - Perhexiline
Absorption
Well absorbed (>80%) from the gastrointestinal tract following oral administration.
The principal metabolites of perhexiline in man are monohydroxyperhexiline (which is excreted, in part, conjugated with glucuronic acid) and dihydroxyperhexiline that accounts for a relatively small proportion of the total metabolites. Two unidentified metabolites have also been found in the faeces. The pharmacological activity of the metabolites is not known. Hydroxylation of perhexiline is controlled by cytochrome P450 2D6 (CY P450 2D6).
Variable and non-linear. Some reports show a half-life of 2-6 days, others indicate it could be as high as 30 days.
Perhexiline binds to the mitochondrial enzyme carnitine palmitoyltransferase (CPT)-1 and CPT-2. It acts by shifting myocardial substrate utilisation from long chain fatty acids to carbohydrates through inhibition of CPT-1 and, to a lesser extent, CPT-2, resulting in increased glucose and lactate utilization. This results in increased ATP production for the same O2 consumption as before and consequently increases myocardial efficiency.
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?