
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
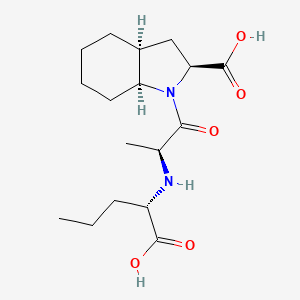

1. S-9780
1. 95153-31-4
2. Perindoprilate
3. Perindoprilato
4. Perindoprilatum
5. S-9780
6. Perindopril Related Compound B
7. Perindopril Diacid Form
8. 2uv6znq92k
9. (2s,3as,7as)-1-((s)-2-(((s)-1-carboxybutyl)amino)propanoyl)octahydro-1h-indole-2-carboxylic Acid
10. (2s,3as,7as)-1-((s)-n-((s)-1-carboxybutyl)alanyl)hexahydro-2-indolinecarboxylic Acid
11. S 9780
12. Perindoprilate [french]
13. Perindoprilatum [latin]
14. Perindoprilato [spanish]
15. (2s,3as,7as)-1-(((s)-1-carboxybutyl)-l-alanyl)octahydro-1h-indole-2-carboxylic Acid
16. Perindoprilat [inn:ban]
17. Unii-2uv6znq92k
18. Brn 4207072
19. Perondropilat
20. Perondroprilat
21. Perindoprilat [inn]
22. Schembl564053
23. Gtpl6373
24. Chembl1201368
25. Dtxsid90869249
26. Chebi:132041
27. Hy-b1433
28. Zinc4217270
29. Perindopril Diacid Form [mi]
30. Akos026750118
31. Ccg-213925
32. Db14213
33. Ncgc00389603-01
34. (2s,3as,7as)-1-[(2s)-2-[[(1s)-1-carboxybutyl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic Acid
35. (2s,3as,7as)-1-[(2s)-2-[[(2s)-1-hydroxy-1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic Acid
36. 1h-indole-2-carboxylic Acid, Octahydro-1-(2-((1-carboxybutyl)amino)-1-oxopropyl)-, (2s-(1(r*(r*)),2-alpha,3a-beta,7a-beta))-
37. Cs-0013140
38. C21517
39. Perindopril Related Compound B [usp-rs]
40. Ab01563365_01
41. 153p314
42. A1-06488
43. Q27088302
44. Perindopril Tert-butylamine Impurity B [ep Impurity]
45. Discontinued. Please See P287531 Or P287535 Or P287585.
46. N-{(2s)-1-[(2s,3as,7as)-2-carboxyoctahydro-1h-indol-1-yl]-1-oxopropan-2-yl}-l-norvaline
47. (2s,3as,7as)-1-((s)-2-(((s)-1-carboxybutyl)amino)propanoyl)octahydro-1h-indole-2-carboxylicacid
48. (2s,3as,7as)-1-[(2s)-2-[[(1s)-1-carboxybutyl]amino]-1-oxopropyl]octahydro-1h-indole-2-carboxylic Acid
49. (2s,3as,7as)-1-[(2s)-2-[[(2s)-1-hydroxy-1-oxopentan-2-yl]amino] Propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic Acid
50. (2s,3as,7as)-1-[(2s)-2-{[(1s)-1-carboxybutyl]amino}propanoyl]octahydro-1h-indole-2-carboxylic Acid
51. (2s,3as,7as)-1-[(2s)-2-{[(1s)-1-carboxybutyl]amino}propanoyl]octahydro-1h-indole-2-carboxylic Acid (non-preferred Name)
52. [2s-[1[r*(r*)],2alpha,3abeta,7abeta]]-1-[2-[(1-carboxybutyl)amino]-1-oxopropyl]octahydro-1h-indole-2-carboxylic Acid
53. X94
| Molecular Weight | 340.4 g/mol |
|---|---|
| Molecular Formula | C17H28N2O5 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 340.19982200 g/mol |
| Monoisotopic Mass | 340.19982200 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 495 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?