
Synopsis
Synopsis
0
JDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
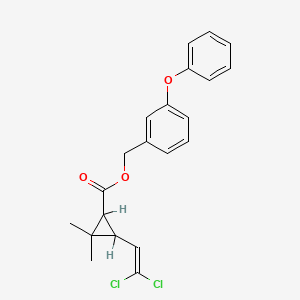

1. (m-phenoxybenzyl)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
2. 3-phenoxybenzyl-(+-)-cis,trans-2,2-dichlorovinyl-2,2-dimethyl-cyclopropylcarboxylic Acid, Ester
3. 3-phenoxybenzyl-cis,trans-(1rs)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
4. Ambush
5. Cis Permethrin
6. Cis-(1rs)-permethrin
7. Cis-permethrin
8. Cyclopropanecarboxylic Acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl Ester
9. Elimite
10. Fmc 33297
11. Fmc-33297
12. Fmc33297
13. Nia 33297
14. Nia-33297
15. Nia33297
16. Nittifor
17. Nrdc 143
18. Nrdc 147
19. Nrdc-143
20. Nrdc-147
21. Nrdc143
22. Nrdc147
23. Permethrin, (1r-cis)-isomer
24. Permethrin, (1r-trans)-isomer
25. Permethrin, (1s-cis)-isomer
26. Permethrin, (1s-trans)-isomer
27. Permethrin, (cis)-isomer
28. Permethrin, (cis-(+-))-isomer
29. Permethrin, (trans)-isomer
30. Permethrin, (trans-(+-))-isomer
31. Permethrin, Cis-(1rs)-isomer
32. Permethrin, Trans-(1rs)-isomer
33. Pp 557
34. Pp-557
35. Pp557
36. S 3151
37. S-3151
38. S3151
39. Trans Permethrin
40. Trans-(1rs)-permethrin
41. Trans-permethrin
1. 52645-53-1
2. Transpermethrin
3. Ambush
4. Pounce
5. Elimite
6. Imperator
7. Nrdc-143
8. (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
9. 1rs,cis-permethrin
10. Transpermethrin [iso]
11. 52341-32-9
12. Hemoglobin Atlanta-coventry
13. Permethrine
14. Permetrina
15. Acticin
16. Ambushfog
17. Corsair
18. Dragnet
19. Ectiban
20. Kaleait
21. Kestrel
22. Outflank
23. Perigen
24. Permasect
25. Perthrine
26. Stomoxin
27. Stomozan
28. Coopex
29. Eksmin
30. Picket
31. Expar
32. Kafil
33. Kavil
34. Anomethrin N
35. Ridect Pour-on
36. Chebi:34911
37. 1rs Cis-permethrin
38. Nsc-760105
39. Ncgc00159390-02
40. 1rs-trans-permethrin
41. Kudos
42. (+-)-cis-permethrin
43. Dsstox_cid_2292
44. 3-phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
45. Trans-(+-)-permethrin
46. Dsstox_rid_76537
47. Dsstox_gsid_22292
48. 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane Carboxylic Acid, (3-phenoxyphenyl) Methyl Ester
49. Permethrinum
50. S-3151
51. Chinetrin
52. Ecsumin
53. Efmethrin
54. Indothrin
55. Lyclear
56. Nrdc 146
57. Nrdc 148
58. Quamlin
59. Stomoxi
60. Cosair
61. Exmin
62. Exsmin
63. Ipitox
64. Sbp-1513
65. (+-)-trans-permethrin
66. Permethrine,c&t
67. (+-)-cis-fmc 33297
68. Diffusil H
69. Insorbcid Mp
70. Stomoxin P
71. Cyclopropanecarboxylic Acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl Ester
72. Outflank-stockade
73. Perigen W
74. Dragnet Ft
75. Picket G
76. Permethrin,racemic
77. [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
78. Mitin Bc
79. Permanone 80
80. Permasect-25ec
81. Fmc 35171
82. 93389-07-2
83. Smr000778043
84. Kestrel (pesticide)
85. Le 79-519
86. Antiborer 3768
87. Cas-52645-53-1
88. Bematin 987
89. Nrdc 143
90. Permethrinum [latin]
91. Permetrin (hungarian)
92. Permitrene (hungarian)
93. Permetrina [portuguese]
94. Caswell No. 652bb
95. Fmc 33297
96. Nia 33297
97. Pp 557
98. Permethrine [iso-french]
99. Brn 4153590
100. Permethrn
101. Sbp-1513tec
102. Permethrin [usan:inn:ban]
103. Unii-509f88p9sz
104. Ai3-29296
105. Ccris 2001
106. Ici-pp 557
107. Mp79
108. Sbp 15131tec
109. 3-phenoxybenzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylate
110. Hsdb 6790
111. M-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
112. Activyl Tick Plus
113. Bw-21-z
114. S 3151
115. 52341-33-0
116. Cyclopropanecarboxylic Acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl Ester, (1r-trans)-
117. Oms 1821
118. Hb Atlanta-coventry
119. Einecs 258-067-9
120. Elimite (tn)
121. Fmc 41655
122. Hb At-co
123. Epa Pesticide Chemical Code 109701
124. Jf 7065
125. Brn 2063148
126. Wl 43479
127. Ai3-29158
128. Permethrin (usan/inn)
129. Chembl1525
130. Schembl26543
131. Cyclopropanecarboxylic Acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl Ester, Cis-(+-)-
132. Cyclopropanecarboxylic Acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl Ester, Trans-(+-)-
133. Mls001332525
134. Mls001332526
135. Permethrin [ansi:bsi:iso]
136. Permethrin Cis/trans ~ 1:1
137. Permethrin, Analytical Standard
138. Dtxsid8022292
139. Schembl15218274
140. 509f88p9sz
141. Hms2232l22
142. Hms3264n07
143. Hms3369d10
144. Pharmakon1600-01504932
145. Cyclopropanecarboxylic Acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl Ester, (1r-cis)-
146. Hy-b0887
147. Tox21_111627
148. Tox21_201586
149. Tox21_300691
150. Mfcd00041809
151. Nsc760105
152. S6461
153. Stl135986
154. Akos005746953
155. Permethrin 100 Microg/ml In Methanol
156. Ccg-213703
157. Db04930
158. Ks-5079
159. Nsc 760105
160. Permethrin 1000 Microg/ml In Acetone
161. (+-)-3-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
162. (3-phenoxyphenyl)methyl 3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropanecarboxylate
163. 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic Acid (3-phenoxyphenyl)methyl Ester
164. 3-phenoxybenzyl (1rs)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
165. 3-phenoxybenzyl (1rs,3rs;1rs,3sr)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
166. 3-phenoxybenzyl(+-)-cis, Trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylate
167. M-phenoxybenzyl (+-)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
168. M-phenoxybenzyl (+1)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
169. Permethrin 10 Microg/ml In Cyclohexane
170. Permethrin 1000 Microg/ml In Methanol
171. Ncgc00159390-00
172. Ncgc00159390-04
173. Ncgc00159390-05
174. Ncgc00159390-06
175. Ncgc00159390-07
176. Ncgc00159390-08
177. Ncgc00159390-09
178. Ncgc00159390-10
179. Ncgc00159390-11
180. Ncgc00159390-12
181. Ncgc00159390-13
182. Ncgc00159390-14
183. Ncgc00254599-01
184. Ncgc00259135-01
185. Permethrin 100 Microg/ml In Cyclohexane
186. Permethrin 1000 Microg/ml In N-hexane
187. (3-phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
188. 3-(phenoxyphenyl)methyl (+-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
189. Permethrin (isomers), Analytical Standard
190. Db-052153
191. Total Permethrin 100 Microg/ml In Acetone
192. Ft-0630656
193. Permethrin, Pestanal(r), Analytical Standard
194. D05443
195. Ab00918441_05
196. Permethrin Is Known As A Pyrethroid Insecticide.
197. 645p531
198. Q411635
199. J-523915
200. Permethrin (25:75), Europepharmacopoeia (ep) Reference Standard
201. (1rs,3sr)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclo-propanecarboxylate
202. 3-phenoxybenzyl 2-(2,2-dichlorovinyl)3,3-dimethylcyclopropanecarboxylate
203. 3-phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
204. (3-phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclopropanecarboxylate
205. 3-phenoxybenzyl (1rs)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate
206. M-phenoxybenzyl 2,2-dimethyl-3-(2',2'-dichlorovinyl)-cyclopropanecarboxylate
207. Permethrin For System Suitability, Europepharmacopoeia (ep) Reference Standard
208. (3-phenoxyphenyl)methyl (+-)cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
209. (3-phenoxyphenyl)methyl (+/-)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate
210. Cyclopropanecarboxylic Acid, 3-(2,2-dichlorovinyl)-2,2-dimethyl-, 3-phenoxybenzyl Ester, (+-)-, (cis,trans)-
211. Permethrin (isomers) Solution, 100 Mug/ml In Acetonitrile, Pestanal(r), Analytical Standard
212. Permethrin (isomers) Solution, Cis/trans Isomers, 1000 Mug/ml In Methanol, Analytical Standard
| Molecular Weight | 391.3 g/mol |
|---|---|
| Molecular Formula | C21H20Cl2O3 |
| XLogP3 | 6.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 390.0789499 g/mol |
| Monoisotopic Mass | 390.0789499 g/mol |
| Topological Polar Surface Area | 35.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 521 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Elimite |
| PubMed Health | Permethrin (On the skin) |
| Drug Classes | Pediculicide, Scabicide |
| Drug Label | ELIMITE (permethrin) 5% Cream is a topical scabicidal agent for the treatment of infestation with Sarcoptesscabiei (scabies). It is available in an off-white, vanishing cream base. ELIMITE (permethrin) 5% Cream is for topical use only.Chemical... |
| Active Ingredient | Permethrin |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Renaissance Pharma |
| 2 of 4 | |
|---|---|
| Drug Name | Permethrin |
| PubMed Health | Permethrin (On the skin) |
| Drug Classes | Pediculicide, Scabicide |
| Drug Label | Permethrin Cream, 5% is a topical scabicidal agent for the treatment of infestation with Sarcoptes scabiei (scabies). It is available in an off-white, vanishing cream base. Permethrin Cream, 5% is for topical use only.Chemical Name The permethrin... |
| Active Ingredient | Permethrin |
| Dosage Form | Cream; Lotion |
| Route | Topical |
| Strength | 1%; 5% |
| Market Status | Over the Counter; Prescription |
| Company | Actavis Mid Atlantic; Perrigo New York |
| 3 of 4 | |
|---|---|
| Drug Name | Elimite |
| PubMed Health | Permethrin (On the skin) |
| Drug Classes | Pediculicide, Scabicide |
| Drug Label | ELIMITE (permethrin) 5% Cream is a topical scabicidal agent for the treatment of infestation with Sarcoptesscabiei (scabies). It is available in an off-white, vanishing cream base. ELIMITE (permethrin) 5% Cream is for topical use only.Chemical... |
| Active Ingredient | Permethrin |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Renaissance Pharma |
| 4 of 4 | |
|---|---|
| Drug Name | Permethrin |
| PubMed Health | Permethrin (On the skin) |
| Drug Classes | Pediculicide, Scabicide |
| Drug Label | Permethrin Cream, 5% is a topical scabicidal agent for the treatment of infestation with Sarcoptes scabiei (scabies). It is available in an off-white, vanishing cream base. Permethrin Cream, 5% is for topical use only.Chemical Name The permethrin... |
| Active Ingredient | Permethrin |
| Dosage Form | Cream; Lotion |
| Route | Topical |
| Strength | 1%; 5% |
| Market Status | Over the Counter; Prescription |
| Company | Actavis Mid Atlantic; Perrigo New York |
MEDICATION (VET)
Crusted (Norwegian) scabies, a rare variant of ordinary scabies, is a highly contagious infection in which the skin is infested with thousands to millions of mites. The infection is frequently overlooked because of its atypical presentations. Patients with cognitive deficiency or an immunodeficiency disorder (including immunosuppressive therapy) are predisposed to developing crusted scabies. The infection often presents as generalized dermatitis with crusted hyperkeratosis on the palms and soles. Diagnosis is made by examining skin scrapings from the crusted lesions. Lindane is the scabicide most widely used in the treatment of crusted scabies. Eradication frequently requires repeated applications, and care must be taken to avoid lindane toxicity. Permethrin cream is as efficacious as lindane in the treatment of ordinary scabies. Because of its wider margin of safety, permethrin may become the preferred treatment for crusted scabies.
PMID:1718155 Kolar KA, Rapini RP; Am Fam Physician 44 (4): 1317-21 (1991)
Permethrin is a synthetic pyrethroid that has low mammalian toxicity and an insecticidal effectiveness higher that of the natural pyrethrins. Because of its high ovicidal activity and persistence on hair, a properly applied 1% cream rinse preparation eliminates head lice infestation after a single application. Fewer than 1% of patients have required retreatment after seven days.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 1436
Permethrin exerts ovicidal effects against lice, and some activity may result from residues that remain on the hair for 2 weeks or longer that kill nymphs as they emerge from eggs.In one in vitro study using live lice and viable nits obtained from healthy lice-infested children in Panama, exposure to permethrin 1% cream rinse killed 30% of the lice within 5 minutes, 53% within 10 minutes, and 100% within 1 hour; 89% of the nits were killed within 10 minutes. However, when permethrin 1% was diluted 10:1 in water to approximate the dilution that occurs when the drug is applied to wet hair, 11% of the lice were killed within 5 minutes, 44% within 10 minutes, and 94% within 1 hour; 81% of the nits were killed within 10 minutes when exposed to the diluted solution.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 84:04.12
For more Therapeutic Uses (Complete) data for PERMETHRIN (19 total), please visit the HSDB record page.
The safety and effectiveness of permethrin in children less than 2 years of age have not been established.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 1436
Patients who cannot tolerate chrysanthemums, pyrethrins, and other synthetic pyrethroids may not tolerate permethrin.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 1436
For the treatment of infestation with Sarcoptes scabiei (scabies).
FDA Label
Treatment of flea infestations (Ctenocephalides felis); the product has persistent insecticidal efficacy for up to 4 weeks against Ctenocephalides felis. The product has persistent acaricidal efficacy for up to 5 weeks against Ixodes ricinus and up to 3 weeks against Rhipicephalus sanguineus. One treatment provides repellent (anti-feeding) activity against sand flies (Phlebotomus perniciosus) for up to 3 weeks.
Permethrin, a pyrethroid, is active against a broad range of pests including lice, ticks, fleas, mites, and other arthropods.
Insecticides
Pesticides designed to control insects that are harmful to man. The insects may be directly harmful, as those acting as disease vectors, or indirectly harmful, as destroyers of crops, food products, or textile fabrics. (See all compounds classified as Insecticides.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
QP53AC54
P03AC04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
P - Antiparasitic products, insecticides and repellents
P03 - Ectoparasiticides, incl. scabicides, insecticides and repellents
P03A - Ectoparasiticides, incl. scabicides
P03AC - Pyrethrines, incl. synthetic compounds
P03AC04 - Permethrin
Absorption
Poorly absorbed through the skin.
Route of Elimination
Permethrin is rapidly metabolized by ester hydrolysis to inactive metabolites which are excreted primarily in the urine.
Lactating cows (three/group) fed permethrin at dose levels of 0, 0.2, 1.0, 10, 50 mg/kg diet for 28 days showed no mortality, and growth and milk production were normal. Permethrin residues were observed in the milk within 3 days at the two highest dietary levels; levels appeared to reach a plateau rapidly and not to increase with time. Analysis of individual cis and trans isomers showed that the ratio of permethrin isomers in milk appeared to change during the course of the study with the cis isomer predominating. Permethrin residues were not found in the tissues of animals that received doses of 1 mg/kg or less. At dose levels of 10 or 50 mg/kg, residues were detected in the tissues, predominantly in the fat. Low levels were also present in the muscle and kidney at the highest dose level. Permethrin did not appear to accumulate in the fat but to reach a plateau rapidly.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
(14)C-cis-Permethrin was applied to the clipped skin of mice at a level of 1 mg/kg body weight in 0.1 mL of acetone. The mice were restrained until the solvent had evaporated and then placed in mouse metabolism cages. They were sacrificed at 1, 5, 15, 50, 480, and 2880 min after treatment and examined for absorption, distribution, and excretion of the insecticide. About 40% of the applied permethrin had moved from the site of application within 5 min and appeared to move rapidly to other parts of the body.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
When ten consecutive oral doses of (14)C-trans- or (14)C-cis- permethrin (labelled in the acid or alcohol moieties) at 0.2-0.3 mg/kg bw/day were given to lactating goats, they excreted 72-79% and 25-36% of the trans and cis isomer doses, respectively, in urine & 12-15%, respectively, in the feces. The amounts of the radiocarbon appearing in the milk were <0.7% with any one of the four (14)C-labelled preparations. Concerning the tissue residues 24 hr after the last dose, detectable levels of radiocarbon were found in most tissue, but none was >0.04 mg/kg for the trans isomer or 0.25 mg/kg for the cis isomer.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
Two human volunteers, who consumed about 2 and 4 mg of permethrin (25:75), respectively, excreted 18-37% and 32-39% of the administered dose, detected as the metabolite Cl2CA, after acid hydrolysis of their urine collected over 24 hr.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
For more Absorption, Distribution and Excretion (Complete) data for PERMETHRIN (37 total), please visit the HSDB record page.
Rapidly metabolized by ester hydrolysis to inactive metabolites which are excreted primarily in the urine.
When the four (14)C-preparations of (IRS)-trans-, (IR)-trans- , (IRS)-cis, and (IR)-cis-permethrin labeled in the alcohol and acid moieties were administered orally to male rats at 1.6-4.8 mg/kg, the compounds were rapidly metabolized and the (14)C from the acid and alcohol moeity was almost completely eliminated from the body within a few days. ... The major metabolic reactions of both permethrin isomers /(trans and cis)/ were oxidation at the trans and cis portions of the gem-dimethyl group of the acid moiety and at the 2'- and 4'-positions of the alcohol moiety, ester cleavage, and the conjugation of the resulting carboxylic acids, alcohols, and phenols with glucuronic acid, glycine, and sulfuric acid. The cis isomer is more stable than the tans isomer, and the cis isomer yielded four fecal ester metabolites which resulted from hydroxylation at the 2'- and 4'-positions of the phenoxy group, at the trans- methyl group, and at both of the two latter sites. The ester-cleaved metabolites were extensively excreted into the urine, whereas the metablites retaining ester linkage were found only in the feces. There were no significant differences in metabolism between the (IRS)-isomers and (IR)-isomers.
Krieger, R. (ed.). Handbook of Pesticide Toxicology. Volume 2, 2nd ed. 2001. Academic Press, San Diego, California., p. 1280
When White Leghorn hens were treated orally three consecutive days with one of four (14)C-trans- and cis-permethrin isomers labelled in the alcohol or acid at 10 mg/kg body weight, they showed no signs of poisoning. More than 87% of the radiocarbon from the four labelled perparations was found in the excreta 9 days after the initial dose, 0.7-4.7% of the dose was exhaled as (14)CO2, and 0.12-0.47% and 0.06-0.66% of the radiocarbon was recovered in egg yolk and fat (subcutaneous and visceral fat), respectively. Both the cis isomers labelled in the alcohol and acid moieties showed recoveries 3 to >10 times higher in the fat and egg yolk than those shown by the corresponding trans isomers. The excreta (0-72 hr) contained 1.7 times more cis-permethrin than trans-permethrin. Hydroxylated ester metabolites of trans-permethrin were not excreted, but four monohydroxy and dihydroxy esters (i.e. trans-OH-permethrin, 4'-OH-permethrin, 4'-OH, trans-OH-permethrin and trans-OH-permethrin sulfate) of cis-permethrin were found. Metabolites from the acid moieties of both isomers were the Cl2CA isomers in free, glucuronide, and taurine conjugate forms, trans-OH-Cl2CA, cis-OH-Cl2CA, cis-OH-Cl2CA lactone, and cis-OH-Cl2CA sulfate. trans-OH-Cl2CA was obtained from the cis isomer to larger extents than from the trans isomer, whereas the amounts of cis-OH-Cl2-CA were larger with the trans isomer than with the cis isomer. The metabolites from the alcohol moiety included PBalc, PBacid, their 4'-hydroxy-derivatives and the corresponding sulfate the glucuronide of PBalc, and a variety of unidentified conjugates of 4'-OH-PBalc and 4'-OH-PBacid. The taurine conjugate of PBacid was not detected. The metabolites produced in largest amounts were the unidentified conjugates of 4'-OH-PBalc (6-13% of the dose) and 4'-OH-PBacid (2-11%). The yolk of eggs 5 and 6 days after initial dosing contained 4.4 times cis-perethrin than trans-permethrin in unchanged form and the same ester metabolites of cis-permethrin as those found in the excreta. Other metabolites in the yolk were generally the same as those in the excreta. Overall, cis-permethrin appeared at higher levels than trans-permethrin in the egg yolk, fatty tissues, and excreta. Radiocarbon from cis-permethrin preparations also persisted longer in the blood than that from trans-permethrin preparations. It probably resulted from more rapid ester cleavage of the trans isomer than the cis isomer, based on the relative amounts of hydrolysis products form the two isomers in hen excreta.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
Two human volunteers, who consumed about 2 and 4 mg of permethrin (25:75), respectively, excreted 18-37% and 32-39% of the administered dose, detected as the metabolite Cl2CA, after acid hydrolysis of their urine collected over 24 hr.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
The permethrin metabolites in goats were formed through cleavage of the ester linkage, hydroxylation at the cis- or trans-methyl of the geminal dimethyl group, and hydroxylation at the 4'-position of the phenoxybenzyl moiety. Some of these metabolic products were further oxidized and/or conjugated with glycine, glutamic acid and glucuronic acid. The major compounds found in feces after dosing with cis-permethrin were unmetabolized parent compound 4'-OH-permethrin, trans-OH-permethrin, PBalc, cis-OH-cis-Cl2CA-lactone and eight unidentified ester metabolites. The feces of goats treated with the trans isomer contained large amounts of the parent compound (41-79% of the fecal (14)C and of PBalc (8-25%) and cis-OH-trans-Cl2CA-lactone. Also, three unidentified ester metabolites were found (8-23%). On the other hand, major urinary metabolites from the alcohol moiety of both isomers were PBacid-glycine (7-9% of the urinary (14)C) and r'-OH-PBacid-glycine (4-12%). PBalc, PBacid, 4'-OH-PBalc, 4'-OH-PBacid, PBacid-glutamic acid and 4'-OH-PBacid-glutamic acid were also identified as minor metabolites. The urine of goats treated with both isomers contained as major components, Cl2CA in the free form (2-4% of the urinary (14)C) and as a glucuronide (27-71%). Cl2CA-glucuronide was obtained to a larger extent with the trans isomer than with the cis isomer. Other major metabolites of the cis isomer were cis-OH-Cl2CA (33) (9-11%) and cis-OH-cis-Cl2CA-lactone (11-16%). trans-OH-Cl2CA was detected as a minor metabolite of both isomers. The milk of goats contained the parent compounds, PBacid-glycine, and 4'-OH-PBacide-glycine. On administration of the cis isomer, a large amount of the parent compund was excreted in the milk than in the case of the trans isomer. Comparatively, when the trans isomer was administered, PBacid-glycine was detected in the milk to a larger extent than with the cis isomer. Most of the radioactivity in the fat was attributed to the parent compound or ester metabolites such as trans-OH-permethrin and trans-OH-permethrin conjugate.
WHO; Environmental Health Criteria 94: Permethrin (1990). Available from, as of August 6, 2014: https://www.inchem.org/pages/ehc.html
For more Metabolism/Metabolites (Complete) data for PERMETHRIN (25 total), please visit the HSDB record page.
The toxicokinetics of permethrin after single 460 mg/kg oral and 46 mg/kg intravenous doses were studied in male Sprague-Dawley rats. Serial blood samples after oral and intravenous dosage, and brain, medulla oblongata, sciatic nerve, and liver samples after oral administration were collected. Plasma, hypothalamus, cerebellum, frontal cortex, caudate putamen, hippocampus, medulla oblongata, sciatic nerve, and liver concentrations of permethrin and its metabolites, m-phenoxybenzyl alcohol and m-phenoxybenzoic acid, were determined by a high-performance liquid chromatographic assay. The permethrin plasma profile could be adequately described by a two-compartment open model. For permethrin, the elimination half-life (t1/2 beta) and the mean residence time from plasma were 8.67 and 11.19 hr after i.v. and 12.37 and 17.77 hr after /peroal/ administration. The total plasma clearance was not influenced by dose concentration or route and reached a value of 0.058 liter/hr. After the single oral dose, permethrin was absorbed slowly with a Tmax of 3.52 hr. The maximum plasma concentration was 49.46 micrograms/ml. The oral bioavailability of permethrin was found to be 60.69%. The plasma concentration-time data for permethrin metabolites as well as the tissue concentration-time data for permethrin and its metabolites after an oral dose of permethrin were found to fit a one-compartment open model. The elimination half-life (t1/2el) of permethrin was greater for the hippocampus, medulla oblongata, frontal cortex, and sciatic nerve (23.10, 22.36, 13.86, and 16.27 hr, respectively) than for plasma (t1/2 beta, 12.37 hr). The maximum amounts of permethrin in cerebellum, hippocampus, caudate putamen, frontal cortex, hypothalamus, and sciatic nerve were about 1.5, 2, 2, 2.7, 4.8, and 7.5 times higher than in plasma, respectively, indicating an accumulation of pyrethroid by nervous tissue itself. Nervous tissue accumulation of permethrin was also reflected by the area under the concentration curve ratios of tissue/plasma (1.16, 3.71, 1.57, 4.27, 3.48, and 8.77, respectively). The metabolites of permethrin, m-phenoxy-benzyl alcohol and m-phenoxybenzoic acid, were detected in plasma and in all selected tissues for 48 hr after dosing, suggesting that a combination of metabolism by the tissues and diffusion into it from the blood may be present.
PMID:1871768 Anadon A et al; Toxicol Appl Pharmacol 110 (1): 1-8 (1991)
In studies, the half-life of (+)-trans- and (+)-cis-permethrin applied to the leaf surface of bean plants was 7 and 9 days, respectively.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 483
Permethrin acts on the nerve cell membrane to disrupt the sodium channel current by which the polarization of the membrane is regulated. Delayed repolarization and paralysis of the pests are the consequences of this disturbance.
Permethrin is pediculocidal by disrupting in sodium channel current in the louse's nerve cell membrane; this action causes delayed polarization of the membrane and paralysis of the insect.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 1436
Like natural pyrethrins, permethrin acts as a neurotoxin by depolarizing nerve cell membranes of parasites. The drug disrupts the sodium channel current by which membrane repolarization is regulated. Delayed repolarization results in paralysis of the nerves in the exoskeletal respiratory muscles of the parasite leading to death.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 84:04.12
The synthetic pyrethroids delay closure of the sodium channel, resulting in a sodium tail current that is characterized by a slow influx of sodium during the end of depolarization. Apparently the pyrethroid molecule holds the activation gate in the open position. Pyrethroids with an alpha-cyano group (e.g., fenvalerate) produce more prolonged sodium tail currents than do other pyrethroids (e.g., permethrin, bioresmethrin). The former group of pyrethroids causes more cutaneous sensations than the latter. /Synthetic pyrethroids/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 1081
Trans- and cis-permethrin were irradiated in sunlight and at gamma>290 nm in hexane, methanol, water and water acetone. Isomerization and ester cleavage occurred primarily. Observed products included monochloropermethrin and the monochlorovinyl acid from cleavage, 3-phenoxybenzyl 3,3-dimethylacrylate, 3-phenoxybenzaldehyde, 3-phenoxybenzoic acid, benzyl alcohol, benzaldehyde, 3-hydroxybenzyl alcohol, benzoic acid, 3-hydroxybenzoic acid, and 3-phenoxybenzyl alcohol.
Menzie, C.M. Metabolism of Pesticides-Update III. Special Scientific Report- Wildlife No. 232. Washington, DC: U.S.Department of the Interior, Fish and Wildlife Service, 1980., p. 484
For more Mechanism of Action (Complete) data for PERMETHRIN (10 total), please visit the HSDB record page.
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
36
PharmaCompass offers a list of Permethrin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Permethrin manufacturer or Permethrin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Permethrin manufacturer or Permethrin supplier.
PharmaCompass also assists you with knowing the Permethrin API Price utilized in the formulation of products. Permethrin API Price is not always fixed or binding as the Permethrin Price is obtained through a variety of data sources. The Permethrin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Permethrin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Permethrin, including repackagers and relabelers. The FDA regulates Permethrin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Permethrin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Permethrin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Permethrin supplier is an individual or a company that provides Permethrin active pharmaceutical ingredient (API) or Permethrin finished formulations upon request. The Permethrin suppliers may include Permethrin API manufacturers, exporters, distributors and traders.
click here to find a list of Permethrin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Permethrin DMF (Drug Master File) is a document detailing the whole manufacturing process of Permethrin active pharmaceutical ingredient (API) in detail. Different forms of Permethrin DMFs exist exist since differing nations have different regulations, such as Permethrin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Permethrin DMF submitted to regulatory agencies in the US is known as a USDMF. Permethrin USDMF includes data on Permethrin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Permethrin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Permethrin suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Permethrin Drug Master File in Korea (Permethrin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Permethrin. The MFDS reviews the Permethrin KDMF as part of the drug registration process and uses the information provided in the Permethrin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Permethrin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Permethrin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Permethrin suppliers with KDMF on PharmaCompass.
A Permethrin CEP of the European Pharmacopoeia monograph is often referred to as a Permethrin Certificate of Suitability (COS). The purpose of a Permethrin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Permethrin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Permethrin to their clients by showing that a Permethrin CEP has been issued for it. The manufacturer submits a Permethrin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Permethrin CEP holder for the record. Additionally, the data presented in the Permethrin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Permethrin DMF.
A Permethrin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Permethrin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Permethrin suppliers with CEP (COS) on PharmaCompass.
A Permethrin written confirmation (Permethrin WC) is an official document issued by a regulatory agency to a Permethrin manufacturer, verifying that the manufacturing facility of a Permethrin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Permethrin APIs or Permethrin finished pharmaceutical products to another nation, regulatory agencies frequently require a Permethrin WC (written confirmation) as part of the regulatory process.
click here to find a list of Permethrin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Permethrin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Permethrin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Permethrin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Permethrin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Permethrin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Permethrin suppliers with NDC on PharmaCompass.
Permethrin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Permethrin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Permethrin GMP manufacturer or Permethrin GMP API supplier for your needs.
A Permethrin CoA (Certificate of Analysis) is a formal document that attests to Permethrin's compliance with Permethrin specifications and serves as a tool for batch-level quality control.
Permethrin CoA mostly includes findings from lab analyses of a specific batch. For each Permethrin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Permethrin may be tested according to a variety of international standards, such as European Pharmacopoeia (Permethrin EP), Permethrin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Permethrin USP).

