
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
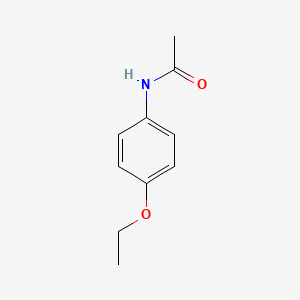

1. Acetophenetidin
1. N-(4-ethoxyphenyl)acetamide
2. 62-44-2
3. Acetophenetidin
4. Acetphenetidin
5. Acetophenetidine
6. Acetophenetin
7. Phenacetine
8. P-acetophenetidide
9. Phenazetin
10. Achrocidin
11. Phenacetinum
12. Fenidina
13. Kalmin
14. 4'-ethoxyacetanilide
15. Contradouleur
16. Codempiral
17. Commotional
18. Contradol
19. 4-ethoxyacetanilide
20. Acetamide, N-(4-ethoxyphenyl)-
21. P-acetophenetidine
22. Fenacetina
23. Pertonal
24. Phenacet
25. Phenacitin
26. Phenedina
27. Phenidin
28. Pyraphen
29. Fenina
30. P-ethoxyacetanilide
31. Phenin
32. P-acetophenetide
33. P-acetphenetidin
34. Phenazetina
35. Tetracydin
36. Clistanol
37. Coriforte
38. Daprisal
39. Dasikon
40. Dolostop
41. Edrisal
42. Empiral
43. Emprazil
44. Epragen
45. Fortacyl
46. Gelonida
47. Gewodin
48. Helvagit
49. Hocophen
50. Melabon
51. Melaforte
52. Pamprin
53. Paramette
54. Paratodol
55. Phenodyne
56. Pyrroxate
57. Quadronal
58. Salgydal
59. Sanalgine
60. Saridon
61. Seranex
62. Sinedal
63. Sinubid
64. Sinutab
65. Stellacyl
66. Synalogos
67. Treupel
68. Veganine
69. Anapac
70. Fenia
71. Malex
72. Tacol
73. Viden
74. Xaril
75. Acetylphenetidin
76. Para-phenacetin
77. Bromo Seltzer
78. Kafa
79. Phenaphen Plus
80. Robaxisal-ph
81. Aceto-4-phenetidine
82. Citra-fort
83. Super Anahist
84. Dasin Ch
85. Emprazil-c
86. Acet-p-phenalide
87. Coryban-d
88. Paracetophenetidin
89. Hjorton's Powder
90. Acet-p-phenetidin
91. Thephorin A-c
92. N-acetyl-p-phenetidine
93. Buff-a-comp
94. Para-acetphenetidin
95. 1-acetamido-4-ethoxybenzene
96. Fiorinal
97. Sinutabs
98. Aceto-para-phenalide
99. Para-acetophenetidide
100. Darvon Compound
101. Acetanilide, 4'-ethoxy-
102. Synalgos-dc
103. P-phenetidine, N-acetyl-
104. Aceto-para-phenetidide
105. Fenacetin [czech]
106. Rcra Waste Number U187
107. Para-acetophenetidine
108. Fenacetin
109. Para-ethoxyacetanilide
110. Fenacetina [inn-spanish]
111. Acetamide, N-(4-ethoxyphenol)-
112. N-para-ethoxyphenylacetamide
113. Ccris 496
114. Hsdb 3152
115. N-acetyl-4-ethoxyaniline
116. P-ethoxyanilid Kyseliny Octove
117. P-ethoxyanilid Kyseliny Octove [czech]
118. Brn 1869238
119. Acetic Acid Amide, N-(4-ethoxyphenyl)-
120. Ai3-00783
121. Nsc-7651
122. 4-(acetylamino)phenetole
123. Er0cth01h9
124. Dolviran
125. 4-ethoxy-1-acetylaminobenzene
126. Chebi:8050
127. 69323-74-6
128. N-[4-(ethyloxy)phenyl]acetamide
129. Phenacetin Melting Point Standard
130. 40674-52-0
131. Cas-62-44-2
132. Ncgc00016281-06
133. Dsstox_cid_1116
134. Dsstox_rid_75948
135. Dsstox_gsid_21116
136. Acetamide, N-(4-ethoxyphenyl)-, Labeled With Tritium
137. N-acetyl-para-phenetidine
138. Phenacetine [inn-french]
139. Phenacetinum [inn-latin]
140. Smr000752916
141. 1-acetyl-p-phenetidin
142. Sr-01000787183
143. Nsc 7651
144. Einecs 200-533-0
145. Rcra Waste No. U187
146. Unii-er0cth01h9
147. Terracydin
148. Phenacetin [usp:inn:jan]
149. N-(4-ethoxyphenyl)-acetamide
150. Zactirin Compound
151. P-acetophenetitide
152. Prestwick_862
153. Phenacetin, 97%
154. 4-ethoxy-acetanilid
155. N-acetylphenetylamine
156. Mfcd00009094
157. 4-ethoxy-acetanilide
158. Asa Compound
159. Butigetic (salt/mix)
160. Spectrum_000782
161. Acetanilide, P-ethoxy-
162. Phenacetin [mi]
163. Phenacetin (jan/inn)
164. Phenacetin-ethoxy-[d5]
165. N-acetyl-p-ethoxyaniline
166. Phenacetin [inn]
167. Phenacetin [jan]
168. Prestwick0_000533
169. Prestwick1_000533
170. Prestwick2_000533
171. Prestwick3_000533
172. Spectrum2_001940
173. Spectrum3_001404
174. Spectrum4_000515
175. Spectrum5_001902
176. Phenacetin [hsdb]
177. Phenacetin [iarc]
178. Phenacetin [inci]
179. P-acetophenetidide (8ci)
180. Phenacetin [vandf]
181. Phenacetinum [hpus]
182. Phenacetin [mart.]
183. Wln: 2or Dmv1
184. Phenacetin [usp-rs]
185. Phenacetin [who-dd]
186. Schembl23280
187. 4'-ethoxyacetanilide, 97%
188. Bspbio_000545
189. Bspbio_003048
190. Kbiogr_001089
191. Kbioss_001262
192. N-(4-ethoxyphenyl)ethanamide
193. Zinc602
194. Mls001304971
195. Mls002153862
196. Mls002303055
197. Chembl16073
198. Divk1c_000580
199. P-a-c Compound (salt/mix)
200. Spectrum1500642
201. Phenacetin [analgesic Mixtures Containing Phenacetin]
202. Spbio_001979
203. Spbio_002466
204. Bpbio1_000601
205. Gtpl7402
206. Dtxsid1021116
207. Schembl20476396
208. Hms501m22
209. Kbio1_000580
210. Kbio2_001262
211. Kbio2_003830
212. Kbio2_006398
213. Kbio3_002268
214. Nsc7651
215. Ninds_000580
216. Hms1569l07
217. Hms1921m21
218. Hms2092e14
219. Hms2096l07
220. Hms2234p11
221. Hms3373i02
222. Hms3651n07
223. Hms3713l07
224. Hms3884h10
225. Pharmakon1600-01500642
226. Bcp09084
227. Hy-b0476
228. Phenacetin, >=98.0% (hplc)
229. Tox21_110347
230. Tox21_201926
231. Tox21_302895
232. Bdbm50420191
233. Ccg-39439
234. Nsc757401
235. Stk011463
236. Akos000370201
237. Phenacetin 1.0 Mg/ml In Acetonitrile
238. Phenacetin 100 Microg/ml In Methanol
239. Tox21_110347_1
240. Db03783
241. Nsc-757401
242. Idi1_000580
243. Acetamide, N-(4-ethoxyphenyl)- (9ci)
244. Ncgc00016281-01
245. Ncgc00016281-02
246. Ncgc00016281-03
247. Ncgc00016281-04
248. Ncgc00016281-05
249. Ncgc00016281-07
250. Ncgc00016281-08
251. Ncgc00016281-11
252. Ncgc00091376-01
253. Ncgc00091376-02
254. Ncgc00091376-03
255. Ncgc00091376-04
256. Ncgc00091376-05
257. Ncgc00256345-01
258. Ncgc00259475-01
259. Ac-28909
260. Sbi-0051571.p002
261. Db-054164
262. Phenacetin, Vetec(tm) Reagent Grade, 98%
263. Ab00052135
264. Ft-0631277
265. Ft-0673664
266. P1669
267. S2577
268. Sw196989-3
269. Bim-0051571.0001
270. C07591
271. D00569
272. D84419
273. Ab00052135_10
274. Ab00052135_11
275. A833774
276. Ae-848/04969036
277. Q419175
278. Phenacetin (136 Degrees C) Melting Point Standard
279. Sr-01000787183-2
280. Sr-01000787183-3
281. Brd-k38323065-001-05-8
282. Brd-k38323065-001-09-0
283. Acetophenetidine Acetophenidin Acetylphenetidine Phenacetin
284. Phenacetin, United States Pharmacopeia (usp) Reference Standard
285. Phenacetin Melting Point Standard, United States Pharmacopeia (usp) Reference Standard
286. N4e
287. Phenacetin Melting Point Standard, Pharmaceutical Secondary Standard; Certified Reference Material
288. Phenacetin Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 179.22 g/mol |
|---|---|
| Molecular Formula | C10H13NO2 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 179.094628657 g/mol |
| Monoisotopic Mass | 179.094628657 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 162 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics, Non-Narcotic; Enzyme Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): Analgesic & antipyretic (eg, for treatment of muscle pain); analgesic & antipyretic in veterinary medicine.
SRI
Medication: It is mainly used for mild to moderate pain associated with the musculo-skeletal system.
GENNARO. REMINGTON'S PHARM SCI 17TH ED 1985 p.1114
The so-called coal tar analgesics, phenacetin and its active metabolite acetaminophen, are effective alternatives to aspirin as analgesic-antipyretics; however, unlike aspirin, their anti-inflammatory activity is weak and seldom clinically useful.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 656
Acetaminophen and phenacetin have analgesic and antipyretic effects that do not differ significantly from those of aspirin. However ... They have only weak anti-inflammatory effects. The pharmacological effects of phenacetin are a combination of its inherent activity and those of acetaminophen, its major metabolite.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 656
Individuals with genetically determined limitation in ability to metabolize phenacetin to acetaminophen convert greater fraction of phenacetin to toxic metabolites, possibly with propensity for serious methemoglobin formation & hemolysis.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 658
Since single 2-g dose of phenacetin in adults converts only 1-3% of total hemoglobin to methemoglobin, methemoglobinemia produced by therapeutic doses... Is not usually of clinical significance. However, in acute overdosage or during chronic abuse, methemoglobinemia may contribute to total toxicity.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 704
Repeated administration is contraindicated in patient with anemia or cardiac, pulmonary, renal, or hepatic disease.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 28:08
Phenacetin has been said to cause relaxation, drowsiness, euphoria, stimulation, & incr efficiency; such effects have been thought to contribute to its abuse liability.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 657
For more Drug Warnings (Complete) data for PHENACETIN (10 total), please visit the HSDB record page.
Used principally as an analgesic.
Phenacetin was the first NSAID and fever reducer to go on the market. It acts as an analgesic at the spinal cord as well as a negative inotrope at the heart. It can be used to treat subacute rheumatoid arthritis, intercostal neuralgia, and ataxias.
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BE - Anilides
N02BE03 - Phenacetin
... Oral absorption of phenacetin is markedly influenced by particle size in the preparation, & plasma concentration of phenacetin & acetaminophen are correspondingly variable.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 703
Peak concentration of phenacetin in plasma usually occurs in about 1 hr, & that of acetaminophen derived there from in 1-2 hr.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 657
Absorption following oral administration is rapid ... duration of effect is about 4 hr.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1060
Up to 45% of (14)C was recovered in 16 hr urine & 1% in feces of rats given [acetyl-(14)C]phenacetin per oral.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 75
For more Absorption, Distribution and Excretion (Complete) data for PHENACETIN (9 total), please visit the HSDB record page.
Metabolised in the body to paracetamol.
Acetaminophen & phenacetin are metabolized primarily by hepatic microsomal enzymes. ... In normal individual, 75 to 80% of administered phenacetin is rapidly metabolized to acetaminophen.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 657
... Phenacetin is converted to at least a dozen other metabolites, by n-deacetylation to para-phenetidin & by hydroxylation & further metabolism of phenacetin & para-phenetidin. An unknown metabolite, but an oxidizing agent, is responsible for methemoglobin formation & hemolysis of red blood cells ... .
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 657
Phenacetin is metabolized ... to p-acetamidophenol, which is excreted as glucuronide and sulfate conjugate ... .
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 176
... N-hydroxyphenacetin has been identified as metabolite in ... man.
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 63
For more Metabolism/Metabolites (Complete) data for PHENACETIN (15 total), please visit the HSDB record page.
Phenacetin has known human metabolites that include N-Hydroxyphenacetin and acetaminophen.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half-life (t1/2)beta varied from 37 to 74 minutes.
PMID:1233222 Raaflaub J et al; Eur J Clin Pharmacol 8 (3-4): 261-5 (1975)
The present study was aimed to test the possible cyclooxygenase (COX)-1/COX-2 selectivity of the old analgesic drug phenacetin and its metabolite p-phenetidine, which exhibits high renal toxicity. Paracetamol (acetaminophen), the main metabolite of phenacetin with low renal toxicity, and indomethacin were selected as reference compounds. Collagen-stimulated platelet thromboxane B2 (TxB2) production and phorbol 12-myristate-13-acetate (PMA)-induced neutrophil prostaglandin E2 (PGE2) synthesis were used as indicators for COX-1 and COX-2 activity, respectively. Phenacetin was even less potent than paracetamol to reduce the production of both TxB2 and PGE2, and no clear preference for either of the COX-enzymes was seen. P-phenetidine was a more potent inhibitor, already at nanomolar level, of the synthesis of these prostanoids than indomethacin and showed some preference to COX-2 inhibition. Somewhat higher, micromolar, concentrations of p-phenetidine also reduced COX-2 expression in neutrophils. We suggest that the very potent inhibitory activity of p-phenetidine on PGE2 synthesis combined with the reduction of COX-2 expression could explain the renal papillary necrosis in phenacetin kidney.
PMID:14592552 Kankuri E et al; Thromb Res 110 (5-6): 299-303 (2003)
Analgesic nephropathy is a unique drug-induced kidney disease characterized pathologically by renal papillary necrosis and chronic interstitial nephritis, and is the result of excessive consumption of combination antipyretic analgesics. The clinical features of the disorder relate mainly to the papillary necrosis, renal colic, and obstructive uropathy and the development of chronic renal failure in a small percentage of patients. There are significant geographic variations in the clinical features that may be related to the differing combinations of analgesics. The pathogenesis of the disease is in part related to the kidneys' ability to concentrate drugs in the papillae. The following sequence of events presents a plausible explanation for the evolution of the disease. If a combination of phenacetin and aspirin is ingested, the following steps occur. Phenacetin is converted in the gut and liver to acetaminophen by first-pass metabolism. Acetaminophen is then taken up by the kidney and excreted. During its excretion, acetaminophen becomes concentrated in the papillae of the kidney during physiologic degrees of antidiuresis, the concentration being up to five times the intracellular concentration of other tissues. Acetaminophen undergoes oxidative metabolism by prostaglandin H synthase to a reactive quinoneimine that is conjugated to glutathione. If acetaminophen is present alone, there is sufficient glutathione generated in the papillae to detoxify the reactive intermediate. If the acetaminophen is ingested with aspirin, the aspirin is converted to salicylate and salicylate becomes highly concentrated in both the cortex and papillae of the kidney. Salicylate is a potent depletor of glutathione. The mechanism is not completely understood; however, the inhibition of the production of NADPH via the pentose shunt is a possible explanation. With the cellular glutathione depleted, the reactive metabolite of acetaminophen then produces lipid peroxides and arylation of tissue proteins, ultimately resulting in necrosis of the papillae.
PMID:8669429 Duggin G; Am J Kidney Dis 28 (1 Suppl 1): S39-47 (1996)
The mechanism of analgesic action has not been fully determined. Acetaminophen may act predominantly by inhibiting prostaglandin synthesis in the central nervous system (CNS) and, to a lesser extent, through peripheral action by blocking pain impulse generation. The peripheral action may also be due to inhibition of of the synthesis or actions of other substances that sensitive pain receptors to mechanical or chemical stimulation. /Acetaminophen/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 16
Acetaminophen probably produces antipyresis by acting centrally on the hypothalamic heat-regulating center to produce peripheral vasodilation resulting in increased blood flow through the skin, sweating, and heat loss. The central action probably involves inhibition of prostaglandin synthesis in the hypothalamus. /Acetaminophen/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 16
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
26
PharmaCompass offers a list of Phenacetin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Phenacetin manufacturer or Phenacetin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Phenacetin manufacturer or Phenacetin supplier.
PharmaCompass also assists you with knowing the Phenacetin API Price utilized in the formulation of products. Phenacetin API Price is not always fixed or binding as the Phenacetin Price is obtained through a variety of data sources. The Phenacetin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Phenacetin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Phenacetin, including repackagers and relabelers. The FDA regulates Phenacetin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Phenacetin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Phenacetin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Phenacetin supplier is an individual or a company that provides Phenacetin active pharmaceutical ingredient (API) or Phenacetin finished formulations upon request. The Phenacetin suppliers may include Phenacetin API manufacturers, exporters, distributors and traders.
click here to find a list of Phenacetin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Phenacetin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Phenacetin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Phenacetin GMP manufacturer or Phenacetin GMP API supplier for your needs.
A Phenacetin CoA (Certificate of Analysis) is a formal document that attests to Phenacetin's compliance with Phenacetin specifications and serves as a tool for batch-level quality control.
Phenacetin CoA mostly includes findings from lab analyses of a specific batch. For each Phenacetin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Phenacetin may be tested according to a variety of international standards, such as European Pharmacopoeia (Phenacetin EP), Phenacetin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Phenacetin USP).
