
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Adipost
2. Bontril
3. Di-ap-trol
4. Dyrexan
5. Hyrex
6. Melfiat
7. Obezine
8. Phendimetrazine Hydrochloride
9. Phendimetrazine Hydrochloride, (2s-trans)-isomer
10. Phendimetrazine Tartrate
11. Phendimetrazine Tartrate, (2s-trans(r-(r*,r*)))-isomer
12. Phendimetrazine Tartrate, (r-(r*,r*))-isomer
13. Phendimetrazine, (2r-cis)-isomer
14. Phendimetrazine, (2s-trans)-isomer
15. Phendimetrazine, Trans(+-)-isomer
16. Prelu-2
17. Trimstat
18. Wehless
19. Weightrol
20. X-trozine
1. Fendimetrazina
2. Mephenmetrazine
3. Antapentan
4. Phendimetrazinum
5. 634-03-7
6. Adphen (base)
7. Phendimetrazine (inn)
8. (2s,3s)-3,4-dimethyl-2-phenylmorpholine
9. Bontril
10. Ab2794w8kv
11. Chebi:8059
12. Nsc 169187
13. Nsc-169187
14. Morpholine, 3,4-dimethyl-2-phenyl-, (2s-trans)-
15. Phendimetrazine [inn]
16. Phendimetrazine [inn:ban]
17. Fendimetrazina [inn-spanish]
18. Morpholine, 3,4-dimethyl-2-phenyl-, (+)-
19. Phendimetrazinum [inn-latin]
20. Hsdb 3381
21. (2r-trans)-3,4-dimethyl-2-phenylmorpholine
22. 3,4-dimethyl-2-phenyltetrahydro-1,4-oxazine
23. Einecs 211-204-6
24. Unii-ab2794w8kv
25. 27032-15-1
26. Morpholine, 3,4-dimethyl-2-phenyl-, (2s,3s)-
27. Morpholine,3,4-dimethyl-2-phenyl-,trans-
28. Phendimetrazine [mi]
29. Phendimetrazine [hsdb]
30. Schembl598950
31. Phendimetrazine [vandf]
32. Chembl1615439
33. Dtxsid1023447
34. Phendimetrazine [who-dd]
35. Zinc22010387
36. Akos006281399
37. Db01579
38. (2s,3s)-3,4-dimethyl-2-phenyl-morpholine
39. C07904
40. D08347
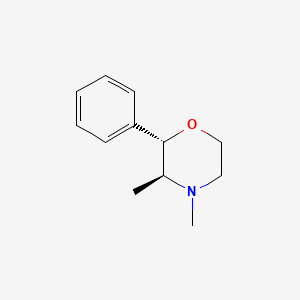
| Molecular Weight | 191.27 g/mol |
|---|---|
| Molecular Formula | C12H17NO |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 191.131014166 g/mol |
| Monoisotopic Mass | 191.131014166 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 177 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Appetite Depressants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... Phendimetrazine /is/ indicated in the short-term (a few weeks) treatment of exogenous obesity in conjunction with a regimen of weight reduction based on caloric restriction, exercise, and behavior modification in patients with a body mass index of > or = 30 kg of body weight per height in meters squared (kg/sq m) or in patients with a body mass index of > or = 27 kg/sq m in the presence of risk factors such as hypertensin and diabetes, or hyperlipidemia. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 438
An increased prevalence of abnormal cardiac valve function, primarily aortic regurgitation, was found on echocardiographic evaluation in patients receiving phentermine in combination with either dexfenfluramine or fenfluramine, both of which act to suppress appetite by increasing serotonergic function; however, an increased prevalence of abnormal cardiac valve function also has been found in patients receiving dexfenfluramine or fenfluramine alone, and the role of phentermine in producing the cardiotoxic effect is uncertain; because of the severity of these cardiovascular effects and because the safety and efficacy of other appetite suppressant combinations have not been established, combined use is not recommended; also, the safety and efficacy of combining as selective serotonin reuptake inhibitor (SSRI), which enhances serotonergic function, with a sympathomimetic appetite suppressant have not been established and combined use is not recommended. /Appetite suppressants, sympathomimetic/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 439
Abnormal heart valve findings and primary pulmonary hypertension have been reported in some patients receiving phendimetrazine tartrate. ... Primary pulmonary hypertension is a rare, frequently fatal pulmonary disease that has been reported with increased frequency in patients receiving anorexigenic agents. One manufacturer of phendimetrazine tartrate ... states that the drug should be used only for short-term management (a few Weeks) of exogenous obesity and should not be used in combination with other anorexigenic agents. /Phendimetrazine tartrate/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2383
Excessive use may lead to tolerance and physical dependence.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 1146
Phendimetrazine is contraindicated in patients with hyperthyroidism, moderate to severe hypertension, advanced arteriosclerosis, symptomatic cardiovascular disease, glaucoma, or known hypersensitivity or idiosyncrasy to sympathomimetic amines. The drug is also contraindicated in patients in agitate states or those who are highly nervous, have a history of drug abuse, or are receiving CNS stimulants. Phendimetrazine is contraindicated during or within 14 days of administration of monoamine oxidase (MAO) inhibitors since hypertensive crisis could result.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2383
For more Drug Warnings (Complete) data for PHENDIMETRAZINE (15 total), please visit the HSDB record page.
Used in the management of exogenous obesity as a short term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction.
Phendimetrazine is a phenylalkylamine sympathomimetic amine with pharmacological activity similar to the prototype drugs of this class used in obesity, the amphetamines. Actions include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance has been demonstrated with all drugs of this class in which these phenomena have been looked for. Drugs of this class used in obesity are commonly known as ''anorectics or anorexigenics." It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions or metabolic effects, may be involved.
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Absorption
Peak plasma levels occur within 1 to 3 hours. Absorption is usually complete by 4 to 6 hours.
Route of Elimination
The major route of elimination is via the kidneys where most of the drug and metabolites are excreted.
Phendimetrazine is readily absorbed from the GI tract and effects persist for about 4 hours after oral administration.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 2383
Approximately 30% of a given dose of phendimetrazine is metabolized into phenmetrazine, which may account for part of its anorectic effect, and probably also influences abuse potential; individuals who metabolise a greater proportion of phendimetrazine into phenmetrazine are more likely to develop problems with dependence and addiction
Phendimetrazine is metabolized in the liver by N-demethylation to the active metabolite phenmetrazine. Phenmetrazine is hydroxylated, conjugated, and excreted in the urine.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 877
19-24 hours
The elimination half life of phendimetrazine is approximately 9 hours for the sustained release and 2 hours for the regular release preparation. The half life of phenmetrazine is approximately 8 hours.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 877
Phendimetrazine may act in a similar way to amphetamines in that it activates the alpha-adrenergic system to induce an appetite suppressive and metabolic increase effect. The drug also acts as a norepinephrine-dopamine releasing agent (NDRA). It can bind to and reverse the NET.
Phendimetrazine is an effective and widely prescribed appetite suppressant. Preclinical findings show that phendimetrazine displays stimulant properties similar to amphetamine, but few studies have examined the neurochemical mechanism of the drug. ... Phendimetrazine itself had no effect on uptake or release of any transmitter. In contrast, the trans-configured N-demethylated metabolite, phenmetrazine, was a potent releaser of [3H]norepinephrine (EC(50)=50 nM) and [3H]dopamine (EC(50)=131 nM). The cis N-demethylated metabolite, pseudophenmetrazine, displayed modest potency at releasing [3H]norepinephrine (EC(50)=514 nM) and blocking [3H]dopamine re-uptake (IC(50)=2630 nM). All drugs tested were inactive or weak in the [3H]5-HT assays. When injected intravenously, phendimetrazine had minimal effects on extracellular transmitter levels, whereas phenmetrazine produced dose-related elevations in extracellular dopamine. The collective findings suggest that phendimetrazine is a "prodrug" that is converted to the active metabolite phenmetrazine, a potent substrate for norepinephrine and dopamine transporters.
PMID:12106802 Rothman RB et al; Eur J Pharmacol ;447 (1): 51-7 (2002)
ABOUT THIS PAGE
76
PharmaCompass offers a list of Phendimetrazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Phendimetrazine manufacturer or Phendimetrazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Phendimetrazine manufacturer or Phendimetrazine supplier.
PharmaCompass also assists you with knowing the Phendimetrazine API Price utilized in the formulation of products. Phendimetrazine API Price is not always fixed or binding as the Phendimetrazine Price is obtained through a variety of data sources. The Phendimetrazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Phendimetrazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Phendimetrazine, including repackagers and relabelers. The FDA regulates Phendimetrazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Phendimetrazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Phendimetrazine supplier is an individual or a company that provides Phendimetrazine active pharmaceutical ingredient (API) or Phendimetrazine finished formulations upon request. The Phendimetrazine suppliers may include Phendimetrazine API manufacturers, exporters, distributors and traders.
Phendimetrazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Phendimetrazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Phendimetrazine GMP manufacturer or Phendimetrazine GMP API supplier for your needs.
A Phendimetrazine CoA (Certificate of Analysis) is a formal document that attests to Phendimetrazine's compliance with Phendimetrazine specifications and serves as a tool for batch-level quality control.
Phendimetrazine CoA mostly includes findings from lab analyses of a specific batch. For each Phendimetrazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Phendimetrazine may be tested according to a variety of international standards, such as European Pharmacopoeia (Phendimetrazine EP), Phendimetrazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Phendimetrazine USP).
