
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
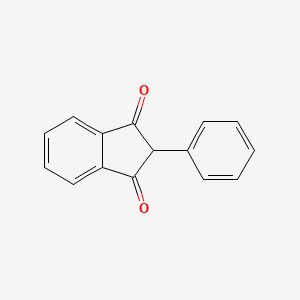

1. 2 Phenyl 1,3 Indandione
2. 2-phenyl-1,3-indandione
3. Dindevan
4. Fenilin
5. Phenylindanedione
6. Phenyline
7. Pindione
1. 83-12-5
2. 2-phenyl-1,3-indandione
3. 2-phenyl-1h-indene-1,3(2h)-dione
4. Rectadione
5. Dindevan
6. Phenylindanedione
7. Phenylindione
8. Danilone
9. Hedulin
10. Athrombon
11. Diophindane
12. Emandione
13. Fenhydren
14. Phenhydren
15. Phenillin
16. Phenyline
17. Theradione
18. Danedion
19. Fenilin
20. Pindione
21. Diadilan
22. Emandion
23. Eridione
24. Fenindion
25. Hemolidione
26. Phenylen
27. Phenylin
28. Tromazal
29. Bindan
30. Dineval
31. Indema
32. Trombol
33. Indon
34. Cronodione
35. Thrombasal
36. Indion
37. 2-phenylindandione
38. 2-phenyl-1,3-diketohydrindene
39. 1h-indene-1,3(2h)-dione, 2-phenyl-
40. 2-phenylindene-1,3-dione
41. Phenindionum
42. Fenindiona
43. Phenyllin
44. 2-phenyl-1,3-indanedione
45. 1,3-indandione, 2-phenyl-
46. 2-phenyl-2,3-dihydro-1h-indene-1,3-dione
47. Pid
48. 2-phenyl-1,3(2h)-indenedione
49. 2-phenylindan-1,3-dione
50. 2-phenylindane-1,3-dione
51. Phenindione-d5
52. Phenindione (inn)
53. Nsc-41693
54. Chembl711
55. Mls000069422
56. Chebi:8066
57. 5m7y6274ze
58. Cas-83-12-5
59. Ncgc00016329-01
60. Smr000059058
61. Phenindione [inn]
62. Dsstox_cid_3453
63. Dsstox_rid_77033
64. Dsstox_gsid_23453
65. Fenindiona [inn-spanish]
66. Phenindionum [inn-latin]
67. Phenylindandione
68. 2-fenyloindandion-1,3
69. 2-fenyloindandion-1,3 [polish]
70. Hedulin (tn)
71. 70711-53-4
72. Hsdb 3155
73. Sr-01000721861
74. Einecs 201-454-4
75. Nsc 41693
76. Phenindione [usp:inn:ban]
77. Unii-5m7y6274ze
78. Prestwick_872
79. Phenindione(rectadione)
80. Spectrum_000927
81. Phenindione (rectadione)
82. Phenindione [mi]
83. Opera_id_1966
84. Prestwick0_000538
85. Prestwick1_000538
86. Prestwick2_000538
87. Prestwick3_000538
88. Spectrum2_000999
89. Spectrum3_000710
90. Spectrum4_000476
91. Spectrum5_001070
92. P1029
93. Rectadione;phenylindandione
94. Phenindione [hsdb]
95. 2-phenyl-indan-1,3-dione
96. Phenindione [mart.]
97. 1h-indene-1, 2-phenyl-
98. Oprea1_684242
99. Schembl33831
100. Bspbio_000555
101. Bspbio_002499
102. Kbiogr_000952
103. Kbioss_001407
104. Phenindione [who-dd]
105. Mls001148439
106. Divk1c_000307
107. Spectrum1500477
108. Spbio_001097
109. Spbio_002476
110. Bpbio1_000611
111. Gtpl6838
112. Dtxsid5023453
113. Hms500p09
114. Kbio1_000307
115. Kbio2_001407
116. Kbio2_003975
117. Kbio2_006543
118. Kbio3_001719
119. Phenindione [orange Book]
120. Ninds_000307
121. Wln: L56 Bv Dv Chj Cr
122. Hms1569l17
123. Hms1920f20
124. Hms2091n22
125. Hms2096l17
126. Hms2234n06
127. Hms3651m04
128. Hms3713l17
129. Pharmakon1600-01500477
130. 2-phenyl-1,3-indandione, 97%
131. Hy-b0325
132. Nsc41693
133. 1,3(2h)-indenedione, 2-phenyl-
134. Tox21_110375
135. Bdbm50280157
136. Ccg-40217
137. Mfcd00003782
138. Nsc757269
139. S1921
140. Stk395038
141. Akos000445019
142. Tox21_110375_1
143. Zinc100004862
144. Db00498
145. Nsc-757269
146. Idi1_000307
147. Ncgc00016329-02
148. Ncgc00016329-03
149. Ncgc00016329-04
150. Ncgc00016329-05
151. Ncgc00016329-07
152. Ncgc00094756-01
153. Ncgc00094756-02
154. Ncgc00094756-03
155. Ncgc00094756-04
156. Bs-18169
157. Sbi-0051480.p003
158. Ab00052069
159. Sw196997-3
160. C07584
161. D08354
162. D92057
163. Ab00052069_13
164. Ab00052069_14
165. Q1640947
166. Sr-01000721861-2
167. Sr-01000721861-3
168. Brd-k70592963-001-21-7
169. Brd-k70592963-001-26-6
170. Phenindione, United States Pharmacopeia (usp) Reference Standard
171. Uas
| Molecular Weight | 222.24 g/mol |
|---|---|
| Molecular Formula | C15H10O2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 222.068079557 g/mol |
| Monoisotopic Mass | 222.068079557 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 304 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticoagulants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...ORAL ANTICOAGULANTS ARE USEFUL IN PREVENTION & TREATMENT OF VARIETY OF THROMBOEMBOLIC DISORDERS. /ORAL ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1362
...INDICATIONS FOR ANTICOAGULANTS...1) MYOCARDIAL INFARCTION, 2) RHEUMATIC HEART DISEASE, 3) CEREBROVASCULAR DISEASE, 4) VENOUS THROMBOSIS & PULMONARY EMBOLISM, & 5) DISSEMINATED INTRAVASCULAR COAGULATION. /ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1363
IT IS INADVISABLE TO CARRY OUT LONG-TERM THERAPY IN CHRONIC ALCOHOLIC, IN INDIVIDUAL WHO MAY REQUIRE INTENSIVE SALICYLATE THERAPY, OR IN CASES OF MALIGNANT HYPERTENSION & ACTIVE TUBERCULOSIS. ORAL ANTICOAGULANT THERAPY DURING PREGNANCY CARRIES SIGNIFICANT HEMORRHAGIC RISK FOR FETUS. /ORAL ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1360
...CONTRAINDICATED IN HEMORRHAGIC TENDENCIES, BLOOD DYSCRASIAS, ULCERATIVE LESIONS OF GI TRACT, DIVERTICULITIS, COLITIS, SUBACUTE BACTERIAL ENDOCARDITIS, THREATENED ABORTION, RECENT OPERATIONS ON BRAIN OR SPINAL CORD. REGIONAL & LUMBAR-BLOCK ANESTHESIA, VITAMIN K DEFICIENCY...HEPATIC OR RENAL DISEASE. /ORAL ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1360
CHANGES IN AVAIL OF VITAMIN K ALTER THERAPEUTIC RESPONSE TO ORAL ANTICOAGULANTS. ... NEWBORN ARE PARTICULARLY SENSITIVE TO ORAL ANTICOAGULANTS. ... RENAL INSUFFICIENCY, FEVER, & SCURVY ENHANCE OR PROLONG ORAL ANTICOAGULANT RESPONSE. /ORAL ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1357
IT CANNOT BE EMPHASIZED TOO STRONGLY THAT TREATMENT OF EACH PT IS HIGHLY INDIVIDUALIZED MATTER. PT ON ANTICOAGULANT THERAPY MUST BE FOLLOWED BY MEANS OF PROTHROMBIN TIME TESTS & OBSERVED CAREFULLY FOR ANY DEVELOPMENT OF BLEEDING TENDENCY. /ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1359
For more Drug Warnings (Complete) data for PHENINDIONE (15 total), please visit the HSDB record page.
For the treatment of pulmonary embolism, cardiomyopathy, atrial fibrillation and flutter, cerebral embolism, mural thrombosis, and thrombophili. Also used for anticoagulant prophylaxis.
Phenindione thins the blood by antagonizing vitamin K which is required for the production of clotting factors in the liver. Anticoagulants such as Phenindione have no direct effect on an established thrombus, nor do they reverse ischemic tissue damage (damage caused by an inadequate blood supply to an organ or part of the body). However, once a thrombus has occurred, the goal of anticoagulant treatment is to prevent further extension of the formed clot and prevent secondary thromboembolic complications which may result in serious and possibly fatal sequelae. Phenindione has actions similar to warfarin, but it is now rarely employed because of its higer incidence of severe adverse effects.
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AA - Vitamin k antagonists
B01AA02 - Phenindione
Absorption
Absorbed slowly from the gastrointestinal tract.
DURATION OF RESPONSE IS DIRECTLY PROPORTIONAL TO T/2 OF DRUG IN PLASMA. LARGER INITIAL DOSE (LOADING DOSE) OF DRUG, SOONER DESIRED THERAPEUTIC RESPONSE IS ATTAINED... /ORAL ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1359
BOTH ITS ONSET & DURATION OF ACTION ARE SHORTER THAN THOSE OF DICUMAROL & WARFARIN. THERAPEUTICALLY EFFECTIVE PROTHROMBIN TIME IS ATTAINED IN 24 TO 48 HR. AFTER DISCONTINUATION OF MAINTENANCE THERAPY, PROTHROMBIN TIME RETURNS TO NORMAL IN 1 TO 4 DAYS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1362
WELL ABSORBED FROM GI TRACT...PEAK PROLONGATION OF PROTHROMBIN TIME 18-48 HR, DURATION 1-4 DAYS...CIRCULATING /DRUG/ ALMOST COMPLETELY BOUND TO PLASMA PROTEINS. UPTAKE...BY ERYTHROCYTES IS VARIABLE...DISTRIBUTED TO LIVER, LUNGS, SPLEEN...KIDNEYS /HUMAN, ORAL/
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1973
Hepatic.
5-10 hours
Phenindione inhibits vitamin K reductase, resulting in depletion of the reduced form of vitamin K (vitamin KH2). As vitamin K is a cofactor for the carboxylation of glutamate residues on the N-terminal regions of vitamin K-dependent proteins, this limits the gamma-carboxylation and subsequent activation of the vitamin K-dependent coagulant proteins. The synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X and anticoagulant proteins C and S is inhibited. Depression of three of the four vitamin K-dependent coagulation factors (factors II, VII, and X) results in decreased prothrombin levels and a decrease in the amount of thrombin generated and bound to fibrin. This reduces the thrombogenicity of clots.
.../ANTICOAGULANTS/ BLOCK HEPATIC FORMATION OF FACTORS II, VII, IX, & X BY COMPETITIVELY INHIBITING ACTION OF VITAMIN K. ... COUMARIN ANTICOAGULANTS MAY ALSO AFFECT TRANSPORT OF VITAMIN K TO ITS SITE OF ACTION. /ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1356
ORAL ANTICOAGULANTS HAVE ONLY ONE MAJOR PHARMACOLOGICAL EFFECT--INHIBITION OF BLOOD-CLOTTING MECHANISMS BY INTERFERING WITH HEPATIC SYNTH OF VITAMIN K-DEPENDENT CLOTTING FACTORS. ...EXERT THEIR INITIAL EFFECT IN VIVO ONLY AFTER LATENT PERIOD... /ORAL ANTICOAGULANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1355
Market Place

ABOUT THIS PAGE
22
PharmaCompass offers a list of Phenindione API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Phenindione manufacturer or Phenindione supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Phenindione manufacturer or Phenindione supplier.
PharmaCompass also assists you with knowing the Phenindione API Price utilized in the formulation of products. Phenindione API Price is not always fixed or binding as the Phenindione Price is obtained through a variety of data sources. The Phenindione Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Phenindione manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Phenindione, including repackagers and relabelers. The FDA regulates Phenindione manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Phenindione API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Phenindione manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Phenindione supplier is an individual or a company that provides Phenindione active pharmaceutical ingredient (API) or Phenindione finished formulations upon request. The Phenindione suppliers may include Phenindione API manufacturers, exporters, distributors and traders.
click here to find a list of Phenindione suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Phenindione Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Phenindione GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Phenindione GMP manufacturer or Phenindione GMP API supplier for your needs.
A Phenindione CoA (Certificate of Analysis) is a formal document that attests to Phenindione's compliance with Phenindione specifications and serves as a tool for batch-level quality control.
Phenindione CoA mostly includes findings from lab analyses of a specific batch. For each Phenindione CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Phenindione may be tested according to a variety of international standards, such as European Pharmacopoeia (Phenindione EP), Phenindione JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Phenindione USP).
