
Synopsis
Synopsis
0
JDMF
0
KDMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
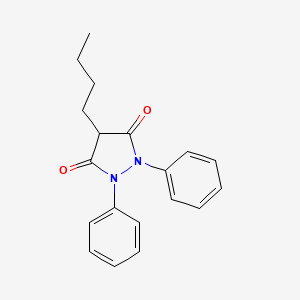

1. Butacote
2. Butadion
3. Butadione
4. Butapirazol
5. Butapyrazole
6. Butazolidin
7. Diphenylbutazone
8. Fenilbutazon
1. 50-33-9
2. 4-butyl-1,2-diphenylpyrazolidine-3,5-dione
3. Butapirazol
4. Butazolidin
5. Butadion
6. Diphenylbutazone
7. Butazolidine
8. Fenibutazona
9. Fenilbutazona
10. Fenylbutazon
11. Intrabutazone
12. Artrizin
13. Butacote
14. Butadiona
15. Diphebuzol
16. Ecobutazone
17. Fenibutol
18. Intrazone
19. Phebuzine
20. Azolid
21. Butoz
22. Buzon
23. Phenylbutazonum
24. Butapyrazole
25. Equipalazone
26. Flexazone
27. Bute
28. Alkabutazona
29. Butapirazole
30. Chembutazone
31. Mephabutazon
32. Mephabutazone
33. Phenylbutazon
34. Praecirheumin
35. Scanbutazone
36. Alqoverin
37. Anpuzone
38. Anuspiramin
39. Arthrizon
40. Artrizone
41. Artropan
42. Azobutyl
43. Bunetzone
44. Butacompren
45. Butagesic
46. Butalgina
47. Butalidon
48. Butarecbon
49. Butartril
50. Butartrina
51. Butazina
52. Butazona
53. Butidiona
54. Butylpyrin
55. Buvetzone
56. Digibutina
57. Diossidone
58. Eributazone
59. Febuzina
60. Fenartil
61. Fenibutal
62. Fenibutasan
63. Fenilbutina
64. Fenilbutine
65. Fenilidina
66. Fenotone
67. Intalbut
68. Ipsoflame
69. Malgesic
70. Merizone
71. Nadazone
72. Nadozone
73. Novophenyl
74. Phebuzin
75. Phenbutazol
76. Phenopyrine
77. Phenylbutaz
78. Phenyzone
79. Pyrabutol
80. Pyrazolidin
81. Rectofasa
82. Reumasyl
83. Reumazin
84. Reumazol
85. Reumuzol
86. Reupolar
87. Robizone
88. Rubatone
89. Zolaphen
90. Alindor
91. Anerval
92. Antadol
93. Benzone
94. Betazed
95. Bizolin
96. Busone
97. Butaluy
98. Butone
99. Diozol
100. Elmedal
101. Lingel
102. Reudox
103. Reumune
104. Kadol
105. Reudo
106. Uzone
107. Butiwas-simple
108. Neo-zoline
109. Phenyl-mobuzon
110. Equi Bute
111. Exrheudon N
112. Robizone-v
113. 4-butyl-1,2-diphenyl-3,5-pyrazolidinedione
114. Robizon-v
115. Butaphen
116. Shigrodin
117. Tevcodyne
118. Azdid
119. Ia-but
120. Pirarreumol B
121. Buta Phen
122. Bizolin 200
123. Phenbutazone
124. Schemergin
125. Butatron
126. Wescozone
127. Tazone
128. Phen-buta-vet
129. 4-butyl-1,2-diphenyl-3,5-dioxopyrazolidine
130. Phenyzene
131. Tencodyne
132. Therazone
133. Todalgil
134. Zolidinum
135. Tetnor
136. Ticinil
137. B.t.z.
138. Mepha-butazon
139. 3,5-pyrazolidinedione, 4-butyl-1,2-diphenyl-
140. 3,5-dioxo-1,2-diphenyl-4-n-butylpyrazolidine
141. 4-butyl-1,2-diphenyl-pyrazolidine-3,5-dione
142. Phenyl Butazone
143. Usaf Ge-15
144. R-3-zon
145. 4-n-butyl-1,2-diphenyl-3,5-pyrazolidinedione
146. Vac-10
147. A 7514
148. Da-192
149. 1,2-diphenyl-4-butyl-3,5-pyrazolidinedione
150. 3,5-dioxo-1,2-diphenyl-4-n-butyl-pyrazolidin
151. G 13,871
152. 1,2-diphenyl-3,5-dioxo-4-butylpyrazolidine
153. 1,2-diphenyl-4-butyl-3,5-dioxopyrazolidine
154. Chebi:48574
155. Fenilbutazon
156. Nsc-25134
157. Gn5p7k3t8s
158. 50-33-9 (free Form)
159. Mls000069424
160. Butadionum
161. Schemergen
162. Nci-c55414
163. Esteve
164. Ncgc00015846-10
165. Ncgc00015846-11
166. Fenilbutazone
167. Smr000059073
168. Fenilbutazone [dcit]
169. Phenylbutazon [german]
170. Dsstox_cid_1136
171. Dsstox_rid_75967
172. Dsstox_gsid_21136
173. G-13871
174. P1z
175. Fenilbutazona [inn-spanish]
176. Phenylbutazonum [inn-latin]
177. Cas-50-33-9
178. Ccris 2374
179. Component Of Azolid-a
180. Hsdb 3159
181. 3,5-dioxe-4 Buty-1, Diphenyl-pyrazolidine
182. Nci-c56531
183. Sr-01000000004
184. Azolid (tn)
185. Einecs 200-029-0
186. Nsc 25134
187. G 13871
188. Unii-gn5p7k3t8s
189. Brn 0290080
190. Alkazone
191. Phenogel
192. Phen-buta
193. Pirarreumol 'b'
194. Phenylbutazone [usp:inn:ban:jan]
195. Mfcd00005500
196. 1,5-dioxopyrazolidine
197. Spectrum_001079
198. 4-butyl-1,5-dione
199. Opera_id_888
200. Spectrum2_001282
201. Spectrum3_000675
202. Spectrum4_000477
203. Spectrum5_001335
204. Lopac-p-8386
205. P1686
206. Epitope Id:124940
207. P 8386
208. Phenylbutazone [mi]
209. Schembl3632
210. Phenylbutazone [inn]
211. Phenylbutazone [jan]
212. Lopac0_000993
213. Oprea1_416494
214. Bspbio_002369
215. Kbiogr_000954
216. Kbioss_001559
217. Phenylbutazone [hsdb]
218. Phenylbutazone [iarc]
219. Mls001148412
220. Mls002152929
221. Divk1c_000331
222. Phenylbutazone [vandf]
223. Spectrum1500482
224. Spbio_001283
225. Phenylbutazone [mart.]
226. 1,5-dioxo-4-butylpyrazolidine
227. 3, 4-butyl-1,2-diphenyl-
228. 4-butyl-1,5-dioxopyrazolidine
229. 4-butyl-1,5-pyrazolidinedione
230. Gtpl7270
231. Phenylbutazone [usp-rs]
232. Phenylbutazone [who-dd]
233. 1,3-dioxo-4-n-butylpyrazoline
234. Dtxsid9021136
235. Hms501a13
236. Kbio1_000331
237. Kbio2_001559
238. Kbio2_004127
239. Kbio2_006695
240. Kbio3_001589
241. Ninds_000331
242. Phenylbutazone (jp17/usp/inn)
243. Bcpp000108
244. Hms1920h06
245. Hms2090h17
246. Hms2091p08
247. Hms2233b13
248. Hms3263g07
249. Hms3369p16
250. Hms3649m17
251. Hms3651g15
252. Hms3715e21
253. Hms3884c06
254. Pharmakon1600-01500482
255. Phenylbutazone, Analytical Standard
256. Phenylbutazone-d10(diphenyl-d10)
257. Component Of Azolid-a (salt/mix)
258. Phenylbutazone [green Book]
259. Bcp02607
260. Hy-b0230
261. Nsc25134
262. 3,2-diphenyl-4-n-butylpyrazolidine
263. Phenylbutazone [orange Book]
264. Tox21_110242
265. Tox21_110243
266. Tox21_201809
267. Tox21_302763
268. Tox21_500993
269. 3,2-diphenyl-4-n-butyl-pyrazolidin
270. Ac-683
271. Ccg-39220
272. Nsc757272
273. Phenylbutazone [ep Monograph]
274. S1654
275. Stk388348
276. Phenylbutazone [usp Monograph]
277. Akos001592731
278. Phenylbutazone (butazolidin, Butatron)
279. Tox21_110242_1
280. Zinc100004227
281. Db00812
282. Ks-5127
283. Lp00993
284. Nsc-757272
285. Sdccgsbi-0050966.p005
286. Wln: T5vnnv Ehj Br& Cr& E4
287. Idi1_000331
288. Ncgc00015846-01
289. Ncgc00015846-02
290. Ncgc00015846-03
291. Ncgc00015846-04
292. Ncgc00015846-05
293. Ncgc00015846-06
294. Ncgc00015846-07
295. Ncgc00015846-08
296. Ncgc00015846-09
297. Ncgc00015846-12
298. Ncgc00015846-13
299. Ncgc00015846-15
300. Ncgc00015846-27
301. Ncgc00023855-03
302. Ncgc00023855-04
303. Ncgc00023855-05
304. Ncgc00023855-06
305. Ncgc00023855-07
306. Ncgc00023855-08
307. Ncgc00181112-01
308. Ncgc00256449-01
309. Ncgc00259358-01
310. Ncgc00261678-01
311. 4297-92-1
312. Bp166190
313. Sbi-0050966.p004
314. Db-051755
315. Suxibuzone Impurity A [ep Impurity]
316. Eu-0100993
317. Ft-0603217
318. Sw199456-2
319. Unm000001255503
320. 1,2-diphenyl-3,5-dioxo-4-butyl-pyrazolidine
321. Phenylbutazone, Saj Special Grade, >=99.0%
322. 1,2-diphenyl-3,5-dioxo-4-n-butylpyrazolidine
323. C07440
324. D00510
325. 1,2-diphenyl-3,5-dioxo-4-n-butyl-pyrazolidine
326. Ab00052071-15
327. Ab00052071-16
328. Ab00052071_17
329. Ab00052071_18
330. A828072
331. Ag-205/04675049
332. Q421342
333. Sr-01000000004-2
334. Sr-01000000004-4
335. Sr-01000000004-5
336. Sr-01000000004-9
337. W-105972
338. Brd-k10843433-001-12-8
339. Brd-k10843433-001-20-1
340. Sr-01000000004-13
341. Z57355370
342. 4-butyl-1,2-diphenyl-1h-pyrazole-3,5(2h,4h)-dione
343. Phenylbutazone, Tracecert(r), Certified Reference Material
344. Phenylbutazone, European Pharmacopoeia (ep) Reference Standard
345. Phenylbutazone, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 308.4 g/mol |
|---|---|
| Molecular Formula | C19H20N2O2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 308.152477885 g/mol |
| Monoisotopic Mass | 308.152477885 g/mol |
| Topological Polar Surface Area | 40.6 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 389 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Phenylbutazone became available for use in humans for the treatment of rheumatoid arthritis and gout in 1949. However, it is no longer approved, and thus not marketed, for any human use in the United States. This is because some patients treated with phenylbutazone have experienced severe toxic reactions, and other effective, less toxic drugs are available to treat the same conditions Phenylbutazone is known to induce blood dyscrasias, including aplastic anemia, leukopenia, agranulocytosis, thrombocytopenia and deaths. Hypersensitivity reactions of the serum-sickness type have also been reported. In addition, phenylbutazone is a carcinogen, as determined by the National Toxicology Program.
FDA; Center for Veterinary Medicine; FDA Order Prohibits Extralabel Use of Phenylbutazone (50-33-9) in Certain Dairy Cattle (February 2003). Available from, as of August 2, 2010: https://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm124078.htm
MEDICATION (Vet): For relief of inflammatory conditions associated with the musculoskeletal system in horses. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Pheynlbutazone injection (May 2010). Available from, as of July 23, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17964
MEDICATION (Vet): Used in vet medicine as analgesic, antipyretic and an anti-inflammatory agent.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 188 (1977)
For more Therapeutic Uses (Complete) data for Phenylbutazone (10 total), please visit the HSDB record page.
Stop medication at the first sign of gastrointestinal upset, jaundice, or blood dyscrasia. Authenticated cases of agranulocytosis associated with the drug have occurred in man. To guard against this possibility, conduct routine blood counts at weekly intervals of two weeks thereafter. Any significant fall in the total white count, relative decrease in granulocytes, or black or tarry stools, should be regarded as a signal for immediate cessation of therapy and institution of appropriate counter measures. In the treatment of inflammatory conditions associated with infections, specific anti-infective therapy is required.
US Natl Inst Health; DailyMed. Current Medication Information for Pheynlbutazone injection (May 2010). Available from, as of July 23, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17964
Treated animals should not be slaughtered for food purposes. Parenteral injections should be made intravenously only; do not inject subcutaneously or intramuscularly. Use with caution in patients who have a history of drug allergy.
US Natl Inst Health; DailyMed. Current Medication Information for Pheynlbutazone injection (May 2010). Available from, as of July 23, 2010 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=17964
The use of phenylbutazone in patients receiving thrombolytic therapy or long-term anticoagulant therapy, therefore, constitutes a serious risk and should be avoided.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1763
Any substantial change in total leukocyte count, relative decrease in granulocytes, appearance of immature blood cells, or a fall in hematocrit or platelet count are indications for immediate discontinuation of phenylbutazone and a complete hematologic evaluation. Hematologic toxicity may occur shortly after initiation of therapy or after prolonged treatment, it may develop abruptly or gradually, and it may become apparent days or weeks following discontinuance of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1761
For more Drug Warnings (Complete) data for Phenylbutazone (31 total), please visit the HSDB record page.
For the treatment of backache and ankylosing spondylitis
Phenylbutazone is a synthetic, pyrazolone derivative. It is a nonhormonal anti-inflammatory, antipyretic compound useful in the management of inflammatory conditions. The apparent analgesic effect is probably related mainly to the compound's anti-inflammatory properties and arise from its ability to reduce production of prostaglandin H and prostacyclin. Prostaglandins act on a variety of cells such as vascular smooth muscle cells causing constriction or dilation, on platelets causing aggregation or disaggregation and on spinal neurons causing pain. Prostacylcin causes vascular constriction platelet disaggregation
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AA - Butylpyrazolidines
M01AA01 - Phenylbutazone
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA01 - Phenylbutazone
Nonsteroidal anti-inflammatory drugs are 95% bound to plasma protein, especially albumin. With extensive protein binding, there is a small volume of distribution (0.10-0.17 L/kg). The pKa for nonsteroidal anti-inflammatory drugs ranges from 3.5 to 5.2.
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 493
Phenylbutazone appears to be rapidly and completely absorbed from the Gl tract. Following oral administration of a single 300 mg dose of phenylbutazone to healthy fasting men, peak plasma phenylbutazone concentrations averaging 43.3 ug/mL are reached within 2.5 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1760
It is recommended that many drugs be taken with or after meals. Recently, however, food intake has been shown to alter significantly the rate and/or extent of absorption of many drugs. Such alterations may induce important changes in the clinical activity of these drugs. Enteric-coated phenylbutazone is recommended to be taken with food to minimize possible gastro-intestinal side-effects. The results of this study demonstrate that while food delays the onset of absorption from this formulation by 4-5 hr, it has no significant effect on the peak concentration or area under the curve. Thus, some effect on fluctuation in plasma levels at steady-state would be expected, but the mean concentration over the recommended dosage interval would remain the same. Treatment efficacy should therefore be unaffected by food but the tolerability may be improved.
PMID:7174832 John VA, Monk JP; J Clin Hosp Pharm 7 (3): 175-9 (1982)
Phenylbutazone is almost completely absorbed after oral administration. A large fraction of the drug in plasma is bound to proteins, and the drug has a small volume of distribution. Phenylbutazone is eliminated by metabolism, only 1% being excreted unchanged in the urine. Approximately 10% of a single dose of phenylbutazone is excreted in bile as metabolites. About 60% of the urinary metabolites have been identified. A novel type of drug metabolite in man, the C-glucuronide, is formed by direct coupling of the pyrazolidine ring of phenylbutazone to glucuronic acid via a C-C bond. Phenylbutazone is oxidised in a phenyl ring or in the side chain to hydroxylated metabolites, which may undergo subsequent O-glucuronidation. After a single dose, C-glucuronidation seems to be the dominant reaction, while oxidation becomes increasingly important after repeated administration. Due to different pharmacokinetic properties of the metabolites, the C-glucuronides are detected in highest concentrations in the urine, while the pharmacologically active compounds oxyphenbutazone and gamma-hydroxyphenbutazone predominate in plasma. The biological (elimination) half-life of phenylbutazone in man is long, with a mean of about 70 hours, and exhibits large interindividual and intraindividual variation. The interindividual variation is largely due to genetic factors.
PMID:359213 Aarbakke J; Clin Pharmacokinet 3 (5): 369-80 (1978)
For more Absorption, Distribution and Excretion (Complete) data for Phenylbutazone (14 total), please visit the HSDB record page.
Phenylbutazone is metabolized in the liver. Phenylbutazone is oxidized to oxyphenbutazone, gamma-hydroxyphenylbutazone, beta-hydroxyphenylbutazone, gamma-ketophenylbutazone, and p,gamma-dihydroxyphenylbutazone. Glucuronide conjugates of phenylbutazone and its metabolites are also formed. In a multiple-dose study in patients with rheumatoid arthritis, plasma concentrations of total oxyphenbutazone decreased with increasing phenylbutazone dose, suggesting that increased chronic doses of phenylbutazone might stimulate elimination of oxyphenbutazone or inhibit oxyphenbutazone formation. Plasma concentrations of gamma-hydroxyphenylbutazone increased proportionally with phenylbutazone dose and showed large interindividual variations.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1760
Major metabolites that have been identified include oxyphenbutazone (ring hydroxylation), Y-hydroxyphenylbutazone (side-chain hydroxylation) Y-hydroxyoxyphenbutazone (dihydroxy metabolite), and 4-hydroxyphenylbutazone. In rats and horses, Y-hydroxyphenylbutazone represents a major (approximately 35%) metabolite and exists in two interchangeable forms; the lactone and the straight-chain forms. The production of the lactone form of Y-phenylbutazone requires cleavage of one of the amide bonds. The formation of this lactone isomer has been shown to be an insignificant reaction in humans. Additional, but apparently minor, products of phenylbutazone oxidation include B-hydroxy- and Y-keto-derivatives of the parent compound.
DHHS/NTP; Toxicology and Carcinogenesis Studies of Phenylbutazone in F344/N Rats and B6C3F1 Mice (Gavage Studies) p.13 (1990) Technical Rpt Series No. 367 NIH Pub No. 90-2822
Phenylbutazone exists in solution in three forms--a diketo, an enol, and a mesomeric anion form. In solution, it exists primarily in the diketo form, and conversion between the forms is slow. These transformations probably contribute to its chemical instability and the ability of the cyclooxygenase system to generate the 4-hydroxphenylbutazone metabolite by a peroxide-dependent cooxygenation reaction.
DHHS/NTP; Toxicology and Carcinogenesis Studies of Phenylbutazone in F344/N Rats and B6C3F1 Mice (Gavage Studies) p.13 (1990) Technical Rpt Series No. 367 NIH Pub No. 90-2822
In addition to the primary metabolites, glucuronide/sulfate conjugates of these primary metabolites have been detected in varying proportions. No glucuronide metabolites have been reported in horses; in rats, approximately 35%-40% of the metabolites are excreted in the urine as conjugated metabolites; in humans, conjugates represent about 50% of urinary metabolites.
DHHS/NTP; Toxicology and Carcinogenesis Studies of Phenylbutazone in F344/N Rats and B6C3F1 Mice (Gavage Studies) p.13 (1990) Technical Rpt Series No. 367 NIH Pub No. 90-2822
... A novel type of drug metabolite in man, the C-glucuronide, is formed by direct coupling of the pyrazolidine ring of phenylbutazone to glucuronic acid via a C-C bond. Phenylbutazone is oxidised in a phenyl ring or in the side chain to hydroxylated metabolites, which may undergo subsequent O-glucuronidation. After a single dose, C-glucuronidation seems to be the dominant reaction, while oxidation becomes increasingly important after repeated administration. Due to different pharmacokinetic properties of the metabolites, the C-glucuronides are detected in highest concentrations in the urine, while the pharmacologically active compounds oxyphenbutazone and gamma-hydroxyphenbutazone predominate in plasma. ...
PMID:359213 Aarbakke J; Clin Pharmacokinet 3 (5): 369-80 (1978)
Biological half-life of phenylbutazone in plasma was about 6 hr in dogs, 5 hr in guinea-pigs and 3 hr in rabbits.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 189
Biological half-life of phenylbutazone in plasma is 72 hours.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V13 190
The plasma half-lives of phenylbutazone and oxyphenbutazone (a metabolite) have been reported to be 50-100 hours with large interindividual and intraindividual variations. The plasma half-life of phenylbutazone has been reported to be shorter in children than in adults and in one study was reported to be about 40 hours in children 1-7 years of age. It was suggested that this may result from enhanced cytochrome p450 enzyme activity in children or a greater liver to body weight ratio in children than in adults. Plasma half-lives of phenylbutazone may be somewhat longer in geriatric patients than in younger adults. Age related biologic and physiologic changes (eg, decreased liver and renal function, decreased serum albumin concentration) may be responsible for altered elimination of the drug in geriatric patients. In patients with severely impaired liver function, plasma phenylbutazone half-lives up to 149 hours have been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1760
After administration of a single dose to humans, the plasma concentration of unaltered drug is characterized by an early maximum of 36 ug/mL at 3 hours and by slow decay between 7 and 336 hours, corresponding to an elimination half-life of 88 hours.
DHHS/NTP; Toxicology and Carcinogenesis Studies of Phenylbutazone in F344/N Rats and B6C3F1 Mice (Gavage Studies) p.12 (1990) Technical Rpt Series No. 367 NIH Pub No. 90-2822
Phenylbutazone binds to and inactivates prostaglandin H synthase and prostacyclin synthase through peroxide (H2O2) mediated deactivation. The reduced production of prostaglandin leads to reduced inflammation of the surrounding tissues.
The drug exhibits anti-inflammatory, analgesic, antipyretic, and mild uricosuric activity. The exact mechanisms have not been clearly established, but many of the actions appear to be associated principally with the inhibition of prostaglandin synthesis. Many nonsteriodal anti- inflammatory agents inhibit the synthesis of prostaglandins in body tissues by inhibiting cyclooxygenase, an enzyme that catalyzes the formation of prostaglandin precursors (endoperoxides) from arachidonic acid.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 1759
Nonsteroidal anti-inflammatory drugs probably act by inhibiting prostaglandin synthesis. Prostaglandins prostaglandins are believed to cause vasodilaton, increased vascular permeability, and increased sensitivity of nerve endings to other inflammatory mediators. By reversibly inhibiting the enzyme cyclooxygenase, nonsteroidal anti-inflammatory drugs block the conversion of the arachidonic acid found in cell membrane phospholipids into varius prostaglandins (E2,F2,D2, thromboxane A2). Since prostaglandins appear to maintain the gastric mucosal barrier nonsteroidal anti-inflammatory drugs inhibition of prostaglandins synthesis may be the cause of the gastritis, peptic ulcerations, and gastrointestinal bleeding observed with nonsteroidal anti-inflammatory drugs. Nonsteroidal anti-inflammatory drugs cause sodium retention, especially in patients with underlying sodium-retaining states such as congestive heart failure. Although the mechanism is not entirely clear, the inhibition of prostaglandins synthesis plays a leading role. These compounds redistribute renal blood flow away from the superficial cortical glomeruli to the juxtamedullary glomeruli, which have a greater capacity to absorb sodium. Stress intensifies the effect of prostaglandins. /Nonsteroidal anti-inflammatory drugs/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 493
Phenylbutazone exists in solution in three forms--a diketo, an enol, and a mesomeric anion form. In solution, it exists primarily in the diketo form, and conversion between the forms is slow. These transformations probably contribute to its chemical instability and the ability of the cyclooxygenase system to generate the 4-hydroxphenylbutazone metabolite by a peroxide-dependent cooxygenation reaction. This reaction has been shown to produce reactive intermediates capable of inactivating prostacyclin synthase and prostaglandin H synthase, which may account for phenylbutazone's anti-inflammatory activity.
DHHS/NTP; Toxicology and Carcinogenesis Studies of Phenylbutazone in F344/N Rats and B6C3F1 Mice (Gavage Studies) p.13 (1990) Technical Rpt Series No. 367 NIH Pub No. 90-2822
Mechanism of anti-inflammatory effects of phenylbutazone is not known. ... Inhibits biosynthesis of prostaglandins, uncouples oxidative phosphorylation, and inhibits ATP-dependent biosynthesis of mucopolysaccharide sulfates in cartilage.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 339

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
97
PharmaCompass offers a list of Phenylbutazone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Phenylbutazone manufacturer or Phenylbutazone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Phenylbutazone manufacturer or Phenylbutazone supplier.
PharmaCompass also assists you with knowing the Phenylbutazone API Price utilized in the formulation of products. Phenylbutazone API Price is not always fixed or binding as the Phenylbutazone Price is obtained through a variety of data sources. The Phenylbutazone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Phenylbutazone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Phenylbutazone, including repackagers and relabelers. The FDA regulates Phenylbutazone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Phenylbutazone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Phenylbutazone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Phenylbutazone supplier is an individual or a company that provides Phenylbutazone active pharmaceutical ingredient (API) or Phenylbutazone finished formulations upon request. The Phenylbutazone suppliers may include Phenylbutazone API manufacturers, exporters, distributors and traders.
click here to find a list of Phenylbutazone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Phenylbutazone DMF (Drug Master File) is a document detailing the whole manufacturing process of Phenylbutazone active pharmaceutical ingredient (API) in detail. Different forms of Phenylbutazone DMFs exist exist since differing nations have different regulations, such as Phenylbutazone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Phenylbutazone DMF submitted to regulatory agencies in the US is known as a USDMF. Phenylbutazone USDMF includes data on Phenylbutazone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Phenylbutazone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Phenylbutazone suppliers with USDMF on PharmaCompass.
A Phenylbutazone CEP of the European Pharmacopoeia monograph is often referred to as a Phenylbutazone Certificate of Suitability (COS). The purpose of a Phenylbutazone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Phenylbutazone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Phenylbutazone to their clients by showing that a Phenylbutazone CEP has been issued for it. The manufacturer submits a Phenylbutazone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Phenylbutazone CEP holder for the record. Additionally, the data presented in the Phenylbutazone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Phenylbutazone DMF.
A Phenylbutazone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Phenylbutazone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Phenylbutazone suppliers with CEP (COS) on PharmaCompass.
A Phenylbutazone written confirmation (Phenylbutazone WC) is an official document issued by a regulatory agency to a Phenylbutazone manufacturer, verifying that the manufacturing facility of a Phenylbutazone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Phenylbutazone APIs or Phenylbutazone finished pharmaceutical products to another nation, regulatory agencies frequently require a Phenylbutazone WC (written confirmation) as part of the regulatory process.
click here to find a list of Phenylbutazone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Phenylbutazone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Phenylbutazone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Phenylbutazone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Phenylbutazone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Phenylbutazone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Phenylbutazone suppliers with NDC on PharmaCompass.
Phenylbutazone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Phenylbutazone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Phenylbutazone GMP manufacturer or Phenylbutazone GMP API supplier for your needs.
A Phenylbutazone CoA (Certificate of Analysis) is a formal document that attests to Phenylbutazone's compliance with Phenylbutazone specifications and serves as a tool for batch-level quality control.
Phenylbutazone CoA mostly includes findings from lab analyses of a specific batch. For each Phenylbutazone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Phenylbutazone may be tested according to a variety of international standards, such as European Pharmacopoeia (Phenylbutazone EP), Phenylbutazone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Phenylbutazone USP).
