
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
Weekly News Recap #Phispers

1. A-1325912.0
2. Abt-530
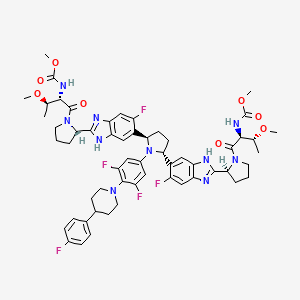
3. Dimethyl N,n'-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis((6-fluoro-1h-benzimidazole-5,2-diyl)((2s)-pyrrolidine-2,1-diyl)((2s,3r)-3-methoxy-1-oxobutane-1,2-diyl)))biscarbamate
1. 1353900-92-1
2. Abt-530
3. Pibrentasvir [usan]
4. Abt 530
5. A-1325912.0
6. Abt530
7. 2wu922tk3l
8. 1353900-92-1 (free)
9. Dimethyl N,n'-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis((6-fluoro-1h-benzimidazole-5,2-diyl)((2s)-pyrrolidine-2,1-diyl)((2s,3r)-3-methoxy-1-oxobutane-1,2-diyl)))biscarbamate
10. Dimethyl ((2s,2's,3r,3'r)-((2s,2's)-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis(6-fluoro-1h-benzo[d]imidazole-5,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methoxy-1-oxobutane-1,2-diyl))dicarbamate
11. Methyl N-[(2s,3r)-1-[(2s)-2-[6-[(2r,5r)-1-[3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl]-5-[6-fluoro-2-[(2s)-1-[(2s,3r)-3-methoxy-2-(methoxycarbonylamino)butanoyl]pyrrolidin-2-yl]-3h-benzimidazol-5-yl]pyrrolidin-2-yl]-5-fluoro-1h-benzimidazol-2-yl]pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl]carbamate
12. Unii-2wu922tk3l
13. Abt-530;pibrentasvir
14. Pibrentasvir [mi]
15. Pibrentasvir(abt-530)
16. Pibrentasvir [inn]
17. Pibrentasvir [jan]
18. Pibrentasvir (abt-530)
19. Pibrentasvir [who-dd]
20. Pibrentasvir (jan/usan/inn)
21. Schembl2756579
22. Chembl3545123
23. Schembl17639956
24. Gtpl11268
25. Ex-a865
26. Dtxsid601027946
27. Pibrentasvir [orange Book]
28. C57h65f5n10o8
29. Bdbm50453100
30. Mavyret Component Pibrentasvir
31. S9641
32. Cs-8135
33. Db13878
34. Ac-33418
35. Bs-15250
36. J3.646.121g
37. D10816
38. J-690144
39. Q47495788
40. A 1325912.0
41. Carbamic Acid, N,n'-(((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)-1-piperidinyl)phenyl)-2,5-pyrrolidinediyl)bis((6-fluoro-1h-benzimidazole-5,2-diyl)-(2s)-2,1-pyrrolidinediyl((1s)-1-((1r)-1-methoxyethyl)-2-oxo-2,1-ethanediyl)))bis-, C,c'-dimethyl Ester
42. Methyl ((2s,3r)-1-((2s)-2-(5-((2r,5r)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)-5-(6-fluoro-2-((2s)-1-(n-(methoxycarbonyl)-o-methyl-l-threonyl)pyrrolidin-2-yl)-1h-benzimidazol-5-yl)pyrrolidin-2-yl)-6-fluoro-1h-benzimidazol-2-yl)pyrrolidin-1-yl)-3-methoxy-1-oxobutan-2-yl)carbamate
| Molecular Weight | 1113.2 g/mol |
|---|---|
| Molecular Formula | C57H65F5N10O8 |
| XLogP3 | 7.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1112.49069988 g/mol |
| Monoisotopic Mass | 1112.49069988 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 80 |
| Formal Charge | 0 |
| Complexity | 2000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A). MAVYRET is also indicated for the treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both.
FDA Label
Pibrentasvir is a pan-genotypic . According to HCV replicon assays, pibrentasvir has EC50 values ranging from 0.08-4.6 nM agaisnt laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4d, 5a, and 6a, or EC50 values of 0.5-4.3 pM against laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4b, 4d, 5a, 6a, 6e and 6p. It is active against common resistance-conferring substitutions in HCV genotypes 1 to 6 that confers resistance and decreased therapeutic response from other NS5A inhibitors, inluding positions 24, 28, 30, 31, 58, 92, or 93 in NS5A. In a QT study, pibrentasvir is not shown to prolong the QTc interval.
Absorption
In healthy subjects, the time it takes to reach the peak plasma concentration (Tmax) is approximately 5 hours. The mean peak plasma concentration (Cmax) is 110ng/mL in non-cirrhotic HCV-infected subjects. Relative to fasting conditions, the consumption of meals increases the absorption of pibrentasvir by 40-53%.
Route of Elimination
The predominant route of elimination of the drug is biliary-fecal, where 96.6% of administered drug is excreted in feces and 0% of the drug is excreted in the urine.
Pibrentasvir is not metabolized.
The elimination half life (t1/2) is approximately 13 hours.
NS5A is a phosphoprotein that plays an essential role in replication, assembly and maturation of infectious viral proteins. The basal phosphorylated form of NS5A, which is maintained by C-terminal serine cluster, is key in ensuring its interaction with the viral capsid protein, or the core protein. By blocking this interaction, pibrentasvir inhibits the assembly of proteins and production of mature HCV particles. NS5A also interacts with viral and cellular proteins to form the HCV replicase complex, and supports the RNA replication of HCV.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
82
PharmaCompass offers a list of Pibrentasvir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pibrentasvir manufacturer or Pibrentasvir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pibrentasvir manufacturer or Pibrentasvir supplier.
PharmaCompass also assists you with knowing the Pibrentasvir API Price utilized in the formulation of products. Pibrentasvir API Price is not always fixed or binding as the Pibrentasvir Price is obtained through a variety of data sources. The Pibrentasvir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pibrentasvir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pibrentasvir, including repackagers and relabelers. The FDA regulates Pibrentasvir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pibrentasvir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pibrentasvir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pibrentasvir supplier is an individual or a company that provides Pibrentasvir active pharmaceutical ingredient (API) or Pibrentasvir finished formulations upon request. The Pibrentasvir suppliers may include Pibrentasvir API manufacturers, exporters, distributors and traders.
click here to find a list of Pibrentasvir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Pibrentasvir Drug Master File in Korea (Pibrentasvir KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Pibrentasvir. The MFDS reviews the Pibrentasvir KDMF as part of the drug registration process and uses the information provided in the Pibrentasvir KDMF to evaluate the safety and efficacy of the drug.
After submitting a Pibrentasvir KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Pibrentasvir API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Pibrentasvir suppliers with KDMF on PharmaCompass.
Pibrentasvir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pibrentasvir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pibrentasvir GMP manufacturer or Pibrentasvir GMP API supplier for your needs.
A Pibrentasvir CoA (Certificate of Analysis) is a formal document that attests to Pibrentasvir's compliance with Pibrentasvir specifications and serves as a tool for batch-level quality control.
Pibrentasvir CoA mostly includes findings from lab analyses of a specific batch. For each Pibrentasvir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pibrentasvir may be tested according to a variety of international standards, such as European Pharmacopoeia (Pibrentasvir EP), Pibrentasvir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pibrentasvir USP).
