

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
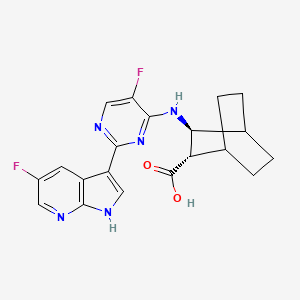

1. (2s,3s)-3-((5-fluoro-2-(5-fluoro-1h-pyrrolo(2,3-b)pyridin-3-yl)-4-pyrimidinyl)amino)bicyclo(2.2.2)octane-2-carboxylic Acid
2. Bicyclo(2.2.2)octane-2-carboxylic Acid, 3-((5-fluoro-2-(5-fluoro-1h-pyrrolo(2,3-b)pyridin-3-yl)-4-pyrimidinyl)amino)-, (2s,3s)-
3. Jnj-63623872
4. Vx-787
1. 1629869-44-8
2. Vx-787
3. Pimodivir [usan]
4. Jnj872
5. Jnj-63623872
6. Vx-787 Anhydrous Base
7. Jnj-63623872-zcd
8. Jnj63623872
9. Dfc121mxc3
10. Chembl3318007
11. Jnj-872
12. (2s,3s)-3-((5-fluoro-2-(5-fluoro-1h-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)bicyclo[2.2.2]octane-2-carboxylic Acid
13. (2s,3s)-3-[[5-fluoranyl-2-(5-fluoranyl-1h-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl]amino]bicyclo[2.2.2]octane-2-carboxylic Acid
14. Bicyclo(2.2.2)octane-2-carboxylic Acid, 3-((5-fluoro-2-(5-fluoro-1h-pyrrolo(2,3-b)pyridin-3-yl)-4-pyrimidinyl)amino)-, (2s,3s)-
15. (2s,3s)-3-[[5-fluoro-2-(5-fluoro-1h-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl]amino]bicyclo[2.2.2]octane-2-carboxylic Acid
16. 21g
17. Pimodivir(vx-787
18. 4p1u
19. Pimodivir [inn]
20. Pimodivir (usan/inn)
21. Pimodivir (vx-787)
22. Unii-dfc121mxc3
23. Pimodivir [who-dd]
24. Schembl2128140
25. Schembl16728380
26. Schembl20150447
27. Dtxsid801028095
28. Ex-a1387
29. Bdbm50050712
30. Hy-12353a
31. Zinc98208077
32. At13207
33. Cs-6423
34. Db14974
35. Vx -787
36. Ncgc00510523-01
37. Ac-30922
38. Jnj - 63623872 - Zcd
39. D11287
40. Q27276370
41. (1r,2s,3s,4r)-3-{[5-fluoro-2-(5-fluoro-1h-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl]amino}bicyclo[2.2.2]octane-2-carboxylic Acid
42. (2s,3s)-3-((5-fluoro-2-(5-fluoro-1h-pyrrolo(2,3-b)pyridin- 3-yl)pyrimidin-4-yl)amino)bicyclo(2.2.2)octane-2-carboxylic Acid
43. (2s,3s)-3-(5-fluoro-2-(5-fluoro-1h-pyrrolo(2,3-b)pyridin-3-yl)pyrimidin-4-ylamino)bicyclo(2.2.2)octane-2-carboxylic Acid
44. 1259366-34-1
45. 3-[[5-fluoro-2-(5-fluoro-1h-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl]amino]bicyclo[2.2.2]octane-2-carboxylic Acid; Vx787; (2s,3s)-3-((5-fluoro-2-(5-fluoro-1h-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)bicyclo[2.2.2]octane-2-carboxylic Acid
| Molecular Weight | 399.4 g/mol |
|---|---|
| Molecular Formula | C20H19F2N5O2 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 399.15068119 g/mol |
| Monoisotopic Mass | 399.15068119 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 620 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of influenza
ABOUT THIS PAGE
We have 1 companies offering Pimodivir
Get in contact with the supplier of your choice:


LOOKING FOR A SUPPLIER?
