
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Antalon
2. Orap
3. Orap Forte
4. R-6238
5. R6238
1. 2062-78-4
2. Orap
3. Opiran
4. Pimozidum
5. Mcn-jr-6238
6. Pimozidum [inn-latin]
7. R-6238
8. R 6238
9. R6238
10. Nsc 170984
11. Mcn-jr 6238
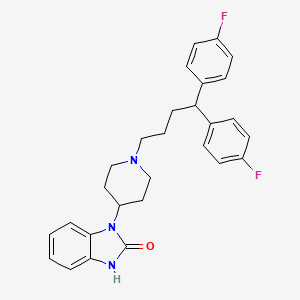
12. 3-[1-[4,4-bis(4-fluorophenyl)butyl]piperidin-4-yl]-1h-benzimidazol-2-one
13. 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2h-benzimidazol-2-one
14. 2h-benzimidazol-2-one, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-
15. Nsc-170984
16. 1-(1-(4,4-bis(p-fluorophenyl)butyl)-4-piperidyl)-2-benzimidazolinone
17. 1-[1-[4,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-2-benzimidazolinone
18. 1hiz4dl86f
19. Chembl1423
20. 1-(4,4-bis(p-fluorophenyl)butyl)-4-(2-oxo-1-benzimidazolinyl)piperidine
21. Mls000028410
22. Mls002702058
23. Chebi:8212
24. Nsc170984
25. 1-{1-[4,4-bis(4-fluorophenyl)butyl]piperidin-4-yl}-1,3-dihydro-2h-benzimidazol-2-one
26. 1-{1-[4,4-bis(4-fluorophenyl)butyl]piperidin-4-yl}-2,3-dihydro-1h-1,3-benzodiazol-2-one
27. 2h-benzimidazol-2-one, 1-(1-(4,4-bis(4-fluorophenyl)butyl)-4-piperidinyl)-1,3-dihydro-
28. Pimozida
29. Ncgc00015802-08
30. Ncgc00015802-18
31. Primozida
32. Smr000058385
33. Cas-2062-78-4
34. R 623
35. R-623
36. Dsstox_cid_3474
37. 1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)-1h-benzo[d]imidazol-2(3h)-one
38. Dsstox_rid_77042
39. Dsstox_gsid_23474
40. Primozida [inn-spanish]
41. Primozide
42. Pimozida [inn-spanish]
43. 1-[4,4-bis(p-fluorophenyl)butyl]-4-(2-oxo-1-benzimidazolinyl)piperidine
44. Ccris 9172
45. Orap (tn)
46. Sr-01000075392
47. Einecs 218-171-7
48. Unii-1hiz4dl86f
49. Brn 0729089
50. 1-(1-(4,4-bis(4-fluorophenyl)butyl)-4-piperidinyl)-1,3-dihydro-2h-benzimidazol-2-one
51. Prestwick_395
52. Mfcd00055081
53. Pimozide [usan:usp:inn:ban:jan]
54. Spectrum_000445
55. Tocris-0937
56. Pimozide [usan]
57. Pimozide [inn]
58. Pimozide [jan]
59. Pimozide [mi]
60. Pimozide [vandf]
61. Opera_id_1580
62. Prestwick0_000308
63. Prestwick1_000308
64. Prestwick2_000308
65. Prestwick3_000308
66. Spectrum2_001026
67. Spectrum3_001451
68. Spectrum4_000420
69. Spectrum5_001308
70. Lopac-p-1793
71. Pimozide [mart.]
72. Gtpl90
73. Pimozide [usp-rs]
74. Pimozide [who-dd]
75. Ncimech_000356
76. P 1793
77. Lopac0_000946
78. Schembl41584
79. Bspbio_000276
80. Bspbio_001439
81. Bspbio_002941
82. Kbiogr_000720
83. Kbioss_000925
84. 5-24-02-00367 (beilstein Handbook Reference)
85. Mls001077311
86. Bidd:gt0435
87. Divk1c_000386
88. Methyl-(2-m-tolylethyl)amine
89. Spectrum1501134
90. Pimozide (jp17/usp/inn)
91. Spbio_001211
92. Spbio_002495
93. Pimozide [orange Book]
94. Bpbio1_000304
95. Schembl7519553
96. Pimozide [ep Monograph]
97. Dtxsid8023474
98. Pimozide [usp Monograph]
99. Bcbcmap01_000043
100. Hms501d08
101. Kbio1_000386
102. Kbio2_000925
103. Kbio2_003493
104. Kbio2_006061
105. Kbio3_002441
106. Ninds_000386
107. Hms1568n18
108. Hms1791h21
109. Hms1921h19
110. Hms1989h21
111. Hms2089c11
112. Hms2092f09
113. Hms2095n18
114. Hms2231p23
115. Hms3262n14
116. Hms3267e19
117. Hms3370n13
118. Hms3402h21
119. Hms3411j16
120. Hms3675j16
121. Hms3712n18
122. Pharmakon1600-01501134
123. Zinc4175630
124. Tox21_110224
125. Tox21_301586
126. Tox21_500946
127. Bdbm50334150
128. Ccg-35918
129. Ccg-36461
130. Ccg-39728
131. Nsc757854
132. S4358
133. 2-benzimidazolinone, 1-[1-[4,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-
134. 3-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidyl]-1h-benzimidazol-2-one
135. Akos024458706
136. Tox21_110224_1
137. Db01100
138. Lp00946
139. Nsc-757854
140. Sdccgsbi-0050920.p004
141. Idi1_000386
142. Smp1_000241
143. Ncgc00015802-01
144. Ncgc00015802-02
145. Ncgc00015802-03
146. Ncgc00015802-04
147. Ncgc00015802-05
148. Ncgc00015802-06
149. Ncgc00015802-07
150. Ncgc00015802-09
151. Ncgc00015802-10
152. Ncgc00015802-11
153. Ncgc00015802-12
154. Ncgc00015802-13
155. Ncgc00015802-14
156. Ncgc00015802-15
157. Ncgc00015802-16
158. Ncgc00015802-26
159. Ncgc00015802-29
160. Ncgc00016601-01
161. Ncgc00022282-03
162. Ncgc00024888-01
163. Ncgc00024888-02
164. Ncgc00024888-03
165. Ncgc00024888-04
166. Ncgc00024888-05
167. Ncgc00024888-06
168. Ncgc00024888-07
169. Ncgc00255294-01
170. Ncgc00261631-01
171. As-13916
172. Hy-12987
173. Sbi-0050920.p003
174. Ab00052215
175. Cs-0012921
176. Eu-0100946
177. Ft-0673903
178. Sw196639-3
179. C07566
180. D00560
181. F70142
182. Ab00052215-06
183. Ab00052215_07
184. Ab00052215_08
185. 062p784
186. L000494
187. Q144085
188. Wln: T56 Bmvnj D- Dt6ntj A3yr Df&r Df
189. J-013477
190. Sr-01000075392-1
191. Sr-01000075392-3
192. Sr-01000075392-4
193. Sr-01000075392-6
194. Brd-k01292756-001-06-0
195. Brd-k01292756-001-13-6
196. Pimozide, European Pharmacopoeia (ep) Reference Standard
197. Z241910386
198. 2-benzimidazolinone,4-bis(p-fluorophenyl)butyl]-4-piperidyl]-
199. N-benzyl-n-(3-isobutoxy-2-(pyrrolidin-1-yl)propyl)benzenamine
200. Pimozide, United States Pharmacopeia (usp) Reference Standard
201. 1-[1-[4,4-bis(p-flurophenyl)butyl]-4-piperidyl]-2-benzimidazolinone
202. 1-(1-(4,4-bis(4-fluorophenyl)butyl)-4-piperidyl)-2-benzimidazolinone
203. 1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)-1,3-dihydro-2h-benzo[d]imidazol-2-one
204. 1-{1-[4,4-bis-(4-fluoro-phenyl)-butyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one
205. 2h-benzimidazol-2-one,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-
206. 1-{1-[4,4-bis-(4-fluoro-phenyl)-butyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one(pimozide)
207. 2h-benzimidazol-2-one, 1-(1-(4,4-bis(4-fluorophenyl)butyl)-4-piperidinyl)-1,3-dihydro
208. Pimozide (1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2h-benzimidaol-2-one)
209. Pimozide1-{1-[4,4-bis-(4-fluoro-phenyl)-butyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one
| Molecular Weight | 461.5 g/mol |
|---|---|
| Molecular Formula | C28H29F2N3O |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 461.22786888 g/mol |
| Monoisotopic Mass | 461.22786888 g/mol |
| Topological Polar Surface Area | 35.6 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 632 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Orap |
| PubMed Health | Pimozide (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | ORAP (pimozide) is an orally active antipsychotic agent of the diphenyl-butylpiperidine series. The structural formula of pimozide, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazole-2-one is:The solubility of pimozide... |
| Active Ingredient | Pimozide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg; 2mg |
| Market Status | Prescription |
| Company | Teva |
| 2 of 2 | |
|---|---|
| Drug Name | Orap |
| PubMed Health | Pimozide (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | ORAP (pimozide) is an orally active antipsychotic agent of the diphenyl-butylpiperidine series. The structural formula of pimozide, 1-[1-[4,4-bis(4-fluorophenyl)butyl]-4-piperidinyl]-1,3-dihydro-2H-benzimidazole-2-one is:The solubility of pimozide... |
| Active Ingredient | Pimozide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 1mg; 2mg |
| Market Status | Prescription |
| Company | Teva |
Used for the suppression of motor and phonic tics in patients with Tourette's Disorder who have failed to respond satisfactorily to standard treatment.
Pimozide is an orally active antipsychotic drug product which shares with other antipsychotics the ability to blockade dopaminergic receptors on neurons in the central nervous system. However, receptor blockade is often accompanied by a series of secondary alterations in central dopamine metabolism and function which may contribute to both pimozide's therapeutic and untoward effects. In addition, pimozide, in common with other antipsychotic drugs, has various effects on other central nervous system receptor systems which are not fully characterized. Pimozide also has less potential for inducing sedation and hypotension as it has more specific dopamine receptor blocking activity than other neuroleptic agents (and is therefore a suitable alternative to haloperidol).
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Anti-Dyskinesia Agents
Drugs used in the treatment of movement disorders. Most of these act centrally on dopaminergic or cholinergic systems. Among the most important clinically are those used for the treatment of Parkinson disease (ANTIPARKINSON AGENTS) and those for the tardive dyskinesias. (See all compounds classified as Anti-Dyskinesia Agents.)
N05AG02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AG - Diphenylbutylpiperidine derivatives
N05AG02 - Pimozide
Absorption
Greater than 50% absorption after oral administration. Serum peak appears 6-8 hours post ingestion.
Notable first-pass metabolism in the liver, primarily by N-dealkylation via the cytochrome P450 isoenzymes CYP3A and CYP1A2 (and possibly CYP2D6). The activity of the two major metabolites has not been determined.
Pimozide has known human metabolites that include 1,3- dihydro-1-(4-piperidinyl)-2H-benzimidazol-2-one (DHPBI).
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
29 ± 10 hours (single-dose study of healthy volunteers).
The ability of pimozide to suppress motor and phonic tics in Tourette's Disorder is thought to be primarily a function of its dopaminergic blocking activity. Pimozide binds and inhibits the dopamine D2 receptor in the CNS.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
49
PharmaCompass offers a list of Pimozide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pimozide manufacturer or Pimozide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pimozide manufacturer or Pimozide supplier.
PharmaCompass also assists you with knowing the Pimozide API Price utilized in the formulation of products. Pimozide API Price is not always fixed or binding as the Pimozide Price is obtained through a variety of data sources. The Pimozide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pimozide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pimozide, including repackagers and relabelers. The FDA regulates Pimozide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pimozide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pimozide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pimozide supplier is an individual or a company that provides Pimozide active pharmaceutical ingredient (API) or Pimozide finished formulations upon request. The Pimozide suppliers may include Pimozide API manufacturers, exporters, distributors and traders.
click here to find a list of Pimozide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pimozide DMF (Drug Master File) is a document detailing the whole manufacturing process of Pimozide active pharmaceutical ingredient (API) in detail. Different forms of Pimozide DMFs exist exist since differing nations have different regulations, such as Pimozide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pimozide DMF submitted to regulatory agencies in the US is known as a USDMF. Pimozide USDMF includes data on Pimozide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pimozide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pimozide suppliers with USDMF on PharmaCompass.
A Pimozide CEP of the European Pharmacopoeia monograph is often referred to as a Pimozide Certificate of Suitability (COS). The purpose of a Pimozide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Pimozide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Pimozide to their clients by showing that a Pimozide CEP has been issued for it. The manufacturer submits a Pimozide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Pimozide CEP holder for the record. Additionally, the data presented in the Pimozide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Pimozide DMF.
A Pimozide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Pimozide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Pimozide suppliers with CEP (COS) on PharmaCompass.
A Pimozide written confirmation (Pimozide WC) is an official document issued by a regulatory agency to a Pimozide manufacturer, verifying that the manufacturing facility of a Pimozide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pimozide APIs or Pimozide finished pharmaceutical products to another nation, regulatory agencies frequently require a Pimozide WC (written confirmation) as part of the regulatory process.
click here to find a list of Pimozide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pimozide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pimozide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pimozide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pimozide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pimozide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pimozide suppliers with NDC on PharmaCompass.
Pimozide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pimozide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pimozide GMP manufacturer or Pimozide GMP API supplier for your needs.
A Pimozide CoA (Certificate of Analysis) is a formal document that attests to Pimozide's compliance with Pimozide specifications and serves as a tool for batch-level quality control.
Pimozide CoA mostly includes findings from lab analyses of a specific batch. For each Pimozide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pimozide may be tested according to a variety of international standards, such as European Pharmacopoeia (Pimozide EP), Pimozide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pimozide USP).
