
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Lb 46
2. Lb-46
3. Lb46
4. Prindolol
5. Visken
1. 13523-86-9
2. Visken
3. Betapindol
4. Prinodolol
5. Carvisken
6. Durapindol
7. Pinbetol
8. Calvisken
9. Decreten
10. Pectobloc
11. Pynastin
12. Lb-46
13. Blocklin L
14. Glauco-visken
15. Pindololum
16. Blocklin-l
17. Dl-pindolol
18. Blockin L
19. Lb 46
20. (rs)-pindolol
21. 1-(1h-indol-4-yloxy)-3-[(1-methylethyl)amino]-2-propanol
22. 4-(2-hydroxy-3-isopropylaminopropoxy)-indole
23. 1-(1h-indol-4-yloxy)-3-(propan-2-ylamino)propan-2-ol
24. 1-(indol-4-yloxy)-3-(isopropylamino)-2-propanol
25. Apo-pindol
26. L-pindolol
27. 1-(1h-indol-4-yloxy)-3-(isopropylamino)propan-2-ol
28. 1-((1-methylethyl)amino)-3-(4-indolyloxy)-2-propanol
29. 4-(3-(isopropylamino)-2-hydroxypropoxy)indole
30. 2-propanol, 1-(1h-indol-4-yloxy)-3-[(1-methylethyl)amino]-
31. Dl-lb-46
32. 1-(1h-indol-4-yloxy)-3-((1-methylethyl)amino)-2-propanol
33. Bj4hf6iu1d
34. Nsc-757276
35. 2-propanol, 1-(4-indolyloxy)-3-(isopropylamino)-
36. Chebi:8214
37. 1-(1h-indol-4-yloxy)-3-[(1-methylethyl)amino]propan-2-ol
38. 2-propanol, 1-(1h-indol-4-yloxy)-3-((1-methylethyl)amino)-
39. Glauco-viskin
40. 1-(1h-indol-4-yloxy)-3-(propan-2-ylamino)-propan-2-ol
41. Ncgc00015786-11
42. 2-propanol, 1-(1h-indol-4-yloxy)-3-(1-methylethyl)amino-
43. (+-)-pindolol
44. Dsstox_cid_3476
45. Pindololum [inn-latin]
46. (-)-pindolol; (s)-(-)-pindolol; S-pindolol
47. Dsstox_rid_77043
48. Dsstox_gsid_23476
49. Dl-4-[2-hydroxy-3-(isopropylamino)propoxy]indole
50. Blocklin
51. Carvisken (tn)
52. Smr000059120
53. Blocklin-l (tn)
54. Visken (tn)
55. Ccris 9215
56. Hsdb 6539
57. Sr-01000000027
58. Einecs 236-867-9
59. Einecs 244-623-8
60. Unii-bj4hf6iu1d
61. Brn 1536506
62. Dl-4-(2-hydroxy-3-(isopropylamino)propoxy)indole
63. Dl-lb 46
64. Prestwick_397
65. Cas-13523-86-9
66. Pindolol [usan:usp:inn:ban:jan]
67. Spectrum_001109
68. (1)-1-(1h-indol-4-yloxy)-3-(isopropylamino)propan-2-ol
69. Pindolol [hsdb]
70. Pindolol [usan]
71. Pindolol [inn]
72. Pindolol [jan]
73. Pindolol [mi]
74. Pindolol [vandf]
75. (.+/-.)-pindolol
76. Prestwick0_000090
77. Prestwick1_000090
78. Prestwick2_000090
79. Prestwick3_000090
80. Spectrum2_001285
81. Spectrum3_000547
82. Spectrum4_000479
83. Spectrum5_001266
84. Pindolol [mart.]
85. Chembl500
86. Gtpl91
87. Pindolol [usp-rs]
88. Pindolol [who-dd]
89. Pindolol,(-)
90. P 0778
91. P-6820
92. Schembl5219
93. Lopac0_000955
94. Oprea1_770884
95. (+/-)-pindolol
96. Bspbio_000020
97. Bspbio_002193
98. Kbiogr_000958
99. Kbioss_001589
100. 5-21-03-00017 (beilstein Handbook Reference)
101. Mls000069496
102. Mls002548891
103. Divk1c_000837
104. Spectrum1500488
105. Pindolol (jp17/usp/inn)
106. Spbio_001289
107. Spbio_001959
108. Pindolol [orange Book]
109. 2-propanol, 1-(indol-4-yloxy)-3-(isopropylamino)-
110. Bpbio1_000022
111. Pindolol [ep Monograph]
112. 1-((1h-indol-4-yl)oxy)-3-(isopropylamino)propan-2-ol
113. Dtxsid8023476
114. Pindolol [usp Monograph]
115. Hms502j19
116. Kbio1_000837
117. Kbio2_001589
118. Kbio2_004157
119. Kbio2_006725
120. Kbio3_001693
121. Ninds_000837
122. 1-(1h-indol-4-yloxy)-3-[(propan-2-yl)amino]propan-2-ol
123. 2-propanol, 1-(indol-4-yloxy)-3-(isopropylamino)-, (+-)-
124. Hms1568a22
125. Hms1920h16
126. Hms2089i21
127. Hms2091p20
128. Hms2095a22
129. Hms3259i07
130. Hms3262p12
131. Hms3267k17
132. Hms3369e14
133. Hms3414j03
134. Hms3678h21
135. Hms3712a22
136. Hms3742c07
137. Hms3885n04
138. Pharmakon1600-01500488
139. Viskazide Component Pindolol
140. Hy-b0982
141. Pindolol, >=98% (tlc), Powder
142. Tox21_110221
143. Tox21_500955
144. Bdbm50019443
145. Ccg-39223
146. Nsc757276
147. Pdsp1_000771
148. Pdsp1_000772
149. Pdsp2_000759
150. Pdsp2_000760
151. (+/-)-pindolol-d7(iso-propyl-d7)
152. Akos015969756
153. Pindolol Component Of Viskazide
154. Tox21_110221_1
155. Cs-4473
156. Db00960
157. Lp00955
158. Nc00467
159. Nsc 757276
160. Sb17015
161. Sdccgsbi-0050929.p005
162. Idi1_000837
163. Ncgc00015786-06
164. Ncgc00015786-07
165. Ncgc00015786-08
166. Ncgc00015786-09
167. Ncgc00015786-10
168. Ncgc00015786-13
169. Ncgc00015786-14
170. Ncgc00015786-16
171. Ncgc00015786-20
172. Ncgc00015786-22
173. Ncgc00024925-03
174. Ncgc00024925-04
175. Ncgc00024925-05
176. Ncgc00024925-06
177. Ncgc00024925-07
178. Ncgc00261640-01
179. Bs-42390
180. Sbi-0050929.p004
181. 4-(3-isopropylamino-2-hydroxypropoxy)indole
182. Ab00052072
183. Eu-0100955
184. Ft-0673907
185. Sw196641-3
186. (+/-)-lb-46
187. C07445
188. C90604
189. D00513
190. Ab00052072-11
191. Ab00052072_12
192. Ab00052072_13
193. 1-(4-indolyloxy)-3-(isopropylamino)-2-propanol
194. 523p869
195. L000006
196. Q418101
197. 4-(2-hydroxy-3-isopropylaminopropoxy)indole
198. Sr-01000000027-2
199. Sr-01000000027-4
200. Sr-01000000027-5
201. Sr-01000000027-7
202. 1-(1h-indol-4-yloxy)-3-isopropylamino-propan-2-ol
203. Brd-a97701745-001-05-3
204. Brd-a97701745-001-09-5
205. 1-(1h-indol-4-yloxy)-3-(isopropylamino)-2-propanol #
206. Pindolol, European Pharmacopoeia (ep) Reference Standard
207. (+/-)-4-(2-hydroxy-3-(isopropylamino)propoxy)indole
208. 1-(1h-indol-4-yloxy)-3-isopropylamino-propan-2-ol(pindolol)
209. Pindolol, United States Pharmacopeia (usp) Reference Standard
210. 1-(1h-indol-4-yloxy)-3-isopropylamino-propan-2-ol((-)-pindolol)
211. N-(2-hydroxy-3-(1h-indol-4-yloxy)propyl)-n-isopropylamine
| Molecular Weight | 248.32 g/mol |
|---|---|
| Molecular Formula | C14H20N2O2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 248.152477885 g/mol |
| Monoisotopic Mass | 248.152477885 g/mol |
| Topological Polar Surface Area | 57.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 248 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Pindolol |
| PubMed Health | Pindolol (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
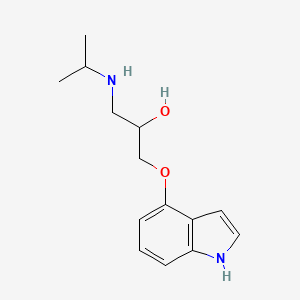
| Drug Label | Pindolol, a synthetic beta-adrenergic receptor blocking agent with intrinsic sympathomimetic activity is 1-(Indol-4-yloxy)-3-(isopropylamino)-2-propanol.C14H20N2O2 M.W. 248.32 Pindolol, USP is a white to off-white, crystalline powder having a f... |
| Active Ingredient | Pindolol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Mutual Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Pindolol |
| PubMed Health | Pindolol (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | Pindolol, a synthetic beta-adrenergic receptor blocking agent with intrinsic sympathomimetic activity is 1-(Indol-4-yloxy)-3-(isopropylamino)-2-propanol.C14H20N2O2 M.W. 248.32 Pindolol, USP is a white to off-white, crystalline powder having a f... |
| Active Ingredient | Pindolol |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 10mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Mutual Pharm |
Adrenergic beta-Antagonists; Anti-Arrhythmia Agents; Antihypertensive Agents; Serotonin Antagonists; Sympatholytics; Sympathomimetics; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A nonselective beta-adrenergic blocking agent with intrinsic sympathomimetic activity that is indicated in the management of hypertension.
Hussar, D.A. (ed.). Modell's Drugs in Current Use and New Drugs. 38th ed. New York, NY: Springer Publishing Co., 1992., p. 131
In the management of hypertension or chronic stable angina pectoris, many clinicians prefer to use low dosages of a beta1-selective adrenergic blocking agent (eg; atenolol, metoprolol), rather than a nonselective agent like pindolol, in patients with chronic obstructive pulmonary disease or insulin-dependent diabetes mellitus. However, selectivity of these agents is relative and dose dependent.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1775
Pindolol ... /is/ indicated in the treatment of classic angina pectoris, also referred to as "effort-associated angina". /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 550
For more Therapeutic Uses (Complete) data for PINDOLOL (6 total), please visit the HSDB record page.
Contraindicted in patients with bronchial asthma, overt cardiac failure, cardiogenic shock, second and third degree heart block, and severe bradycardia. Adverse reactions include dizziness, fatigue, insomnia, edema, nausea, and muscle and joint pain. Use is best avoided in patients with bronchospastic diseases and therapy in diabetic patients must be closely monitored. When therapy is to be discontinued, the dosage should be gradually reduced over a period of 1 to 2 weeks.
Hussar, D.A. (ed.). Modell's Drugs in Current Use and New Drugs. 38th ed. New York, NY: Springer Publishing Co., 1992., p. 131
Abrupt withdrawal of pindolol may exacerbate angina symptoms or precipitate myocardial infarction in patients with coronary artery disease, or precipitate thyroid crisis in patients with thyrotoxicosis. Therefore, patients receiving pindolol (especially those with ischemic heart disease) should be warned not to interrupt or discontinue therapy without consulting their physician. When discontinuance of long term pindolol therapy is planned, particularly in patients with ischemic heart disease, dosage of the drug should be gradually reduced over a period of 1-2 wk. When pindolol therapy is discontinued, patients should be carefully monitored. If exacerbation of angina occurs or acute coronary insufficiency develops after pindolol therapy is interrupted or discontinued, treatment with the drug should be reinstituted promptly, at least temporarily, and appropriate measures for the management of unstable angina pectoris should be initiated. Because coronary artery disease is common and may be unrecognized, the manufacturers caution that it may be prudent not to discontinue pindolol therapy abruptly, even in patients being treated only for hypertension.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1777
Pindolol should be used with caution in patients undergoing major surgery involving general anesthesia. The necessity of withdrawing beta-adrenergic blocking therapy prior to major surgery is controversial, but the manufacturers state that, if possible, pindolol should be withdrawn well before surgery. Severe, protracted hypotension and difficulty in restarting or maintaining a heart beat have occurred during surgery in some patients who have received beta-adrenergic blocking agents. If patients continue to receive pindolol prior to surgery, the anesthesiologist should be advised that the patient is receiving the drug. The manufacturers recommend administration of beta-agonists (eg; dopamine, dobutamine, isoproterenol) to reverse pindolol's beta-adrenergic blockade if necessary during surgery.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1777
Pindolol should be used with caution and in reduced dosage in patients with impaired hepatic function.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1777
For more Drug Warnings (Complete) data for PINDOLOL (14 total), please visit the HSDB record page.
Pindolol is indicated in the management of hypertension. In Canada, it is also indicated in the prophylaxis of angina.
Pindolol is a nonselective beta blocker indicated in the management of hypertension and prophylaxis of angina. It has a short duration of action as it is given twice daily, and a wide therapeutic window as doses can range from 10-60 mg/day. Patients should be counselled regarding the risk of cardiac failure, exacerbating ischemic heart disease with sudden withdrawal, nonallergic bronchospasm, masking hypoglycemia in diabetics, and masking hyperthyroidism.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
Adrenergic beta-Antagonists
Drugs that bind to but do not activate beta-adrenergic receptors thereby blocking the actions of beta-adrenergic agonists. Adrenergic beta-antagonists are used for treatment of hypertension, cardiac arrhythmias, angina pectoris, glaucoma, migraine headaches, and anxiety. (See all compounds classified as Adrenergic beta-Antagonists.)
C - Cardiovascular system
C07 - Beta blocking agents
C07A - Beta blocking agents
C07AA - Beta blocking agents, non-selective
C07AA03 - Pindolol
Absorption
The mean oral bioavailability of pindolol is 87-92%. A 5 mg oral dose reaches a Cmax of 33.1 5.2 ng/mL, with a Tmax of 1-2 hours.
Route of Elimination
80% of an oral dose is eliminated in the urine, with 25-40% of the dose as the unchanged parent compound. 6-9% of an intravenous dose is eliminated in the feces. Overall, 60-65% of a dose is eliminated as glucuronide and sulfate metabolites.
Volume of Distribution
The volume of distribution of pindolol is approximately 2-3 L/kg.
Clearance
In otherwise healthy patients, the systemic clearance of pindolol is 400-500 mL/min. In patients with cirrhosis, the clearance of pindolol varies from 50-300 mL/min.
Pindolol is rapidly absorbed from the GI tract. Reported bioavailability ranges from 50-95%; bioavailability in uremic patients may be at the lower end of this range. Food does not reduce bioavailability but may increase the rate of GI absorption. Pindolol reportedly does not undergo substantial metabolism on first pass through the liver; ... only about 20% of an oral dose is metabolized on first pass. Peak plasma concentrations of 45-167 ng/ml are reached within 1-2 hr after administration of a single 20-mg dose. The extent of absorption may be decreased in patients with impaired renal function. The effect of pindolol on heart rate usually is seen within 3 hr and acute hemodynamic effects persist for 24 hr after administration of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
Approximately 40-60% of pindolol is bound to plasma proteins. In healthy adults, the drug has an apparent volume of distribution of 1.2-2 l/kg; volume of distribution may be decreased by 50% in uremic patients. Pindolol is distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
Elimination of pindolol appears to be a first-order process over a dose range of 5-20 mg. The drug has a plasma half-life of 3-4 hr in healthy adults. Plasma half-life increases to 3-11.5 hr in patients with renal failure, to 7-15 hr in geriatric patients, and varies from 2.5-30 hr in patients with hepatic cirrhosis. Approximately 60-65% of pindolol is metabolized in the liver to hydroxylated metabolites which are then excreted in urine as glucuronides and ethereal sulfates. In healthy adults, about 35-50% of the drug is excreted in urine unchanged; in patients with creatinine clearances less than 20 ml/min, less than 15% is excreted in urine unchanged.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
Pindolol is rapidly and reproducibly absorbed (greater than 95%), achieving peak concentrations within 1 hr of drug administration ... The blood concentrations are proportional in a linear manner to the administered dose in the range of 5-20 mg ... Pindolol is only 40% bound to plasma proteins and is evenly distributed between plasma and red cells. The volume of distribution in healthy subjects is about 2 L/kg.
PDR; Physicians' Desk Reference Generics. 2nd Ed. Montvale, NJ: Medical Economics Co. p. 2489 (1996)
The polar metabolites are excreted with a half-life of approximately 8 hr and thus multiple dosing therapy ... results in a less than 50% accumulation in plasma. About 6%-9% of an administered intravenous dose is excreted by the bile into the feces.
PDR; Physicians' Desk Reference Generics. 2nd Ed. Montvale, NJ: Medical Economics Co. p. 2488 (1996)
30-40% of a dose of pindolol is not metabolized. The remainder is hydroxylated and subsequently undergoes glucuronidation or sulfate conjugation.
Pindolol reportedly does not undergo substantial metabolism on first pass through the liver; ... only about 20% of an oral dose is metabolized on first pass.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
Approximately 60-65% of pindolol is metabolized in the liver to hydroxylated metabolites which are then excreted in urine as glucuronides and ethereal sulfates.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
The half life of pindolol varies from 3-4 hours but can be as high as 30 hours in patients with cirrhosis of the liver.
The drug has a plasma half-life of 3-4 hr in healthy adults. Plasma half-life increases to 3-11.5 hr in patients with renal failure, to 7-15 hr in geriatric patients, and varies from 2.5-30 hr in patients with hepatic cirrhosis
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
The beta-1 adrenoceptor is a G-protein-coupled receptor. Agonism of the beta-1 adrenoceptor allows the Gs subunit to upregulate adenylyl cyclase, converting ATP to cyclic AMP (cAMP). Increased concentrations of cAMP activate cAMP-dependant kinase A, phosphorylating calcium channels, raising intracellular calcium, increasing calcium exchange through the sarcoplasmic reticulum, and increasing cardiac inotropy. cAMP-dependant kinase A also phosphorylates myosin light chains, increasing smooth muscle contractility. Increased smooth muscle contractility in the kidney releases renin. Pindolol is a non-selective beta blocker. Blocking beta-1 adrenergic receptors in the heart results in decreased heart rate and blood pressure. By blocking beta-1 receptors in the juxtaglomerular apparatus, pindolol inhibits the release of renin, which inhibits angiotensin II and aldosterone release. Reduced angiotensin II inhibits vasoconstriction and reduced aldosterone inhibits water retention. Beta-2 adrenoceptors located in the kidneys and peripheral blood vessels use a similar mechanism to activate cAMP-dependant kinase A to increase smooth muscle contractility. Blocking of the beta-2 adrenoceptor relaxes smooth muscle, leading to vasodilation.
Class II antiarrhythmic, beta-adrenoceptor-blocking agents are membrane- depressant drugs that decrease the influx of sodium and calcium ions by reducing membrane-bound adenylate cyclase and cAMP. The reduction in cation transport lengthens depolarization by decreasing the amplitude and slope of the transmembrane potential. Beta-adrenoceptor-blocking agents also reduce myocardial contractility by decreasing calcium release from the sarcoplasmic reticulum (the influx of calcium couples excitation and contraction by initiating the release of calcium from the sarcoplasmic reticulum). Membrane-stabilizing activity enhances the reduction in myocardial contractility in overdose with these drugs in addition to producing a quinidinelike effect of QRS widening. A direct myocardial effect also leads to myocardial depression independent of beta-adrenergic blockade and membrane stabilization. /Class II beta-Blockers/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 191
The principal physiologic action of pindolol is to competitively block beta-adrenergic receptors within the myocardium (beta1-receptors) and within bronchial and vascular smooth muscle (beta2-receptors). In addition to inhibiting access of physiologic or synthetic catecholamines to the beta-adrenergic receptors, pindolol causes slight activation of the beta-receptors, making the drug a partial beta-agonist. This intrinsic sympathomimetic activity of pindolol differs from the beta-agonist activity of epinephrine and isoproterenol in that the maximum beta-adrenergic stimulation that can be obtained with pindolol is less. Other beta-adrenergic blocking agents can block pindolol's intrinsic sympathomimetic activity. Pindolol also has been shown to possess membrane-stabilizing activity or a quinidine-like effect, but this occurs only at plasma concentrations well above those obtained therapeutically. Unlike atenolol and metoprolol, pindolol is not a beta1-selective adrenergic blocking agent; pindolol is a nonselective beta-adrenergic blocking agent, inhibiting both beta1- and beta2-adrenergic receptors.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1777
By inhibiting myocardial beta1-adrenergic receptors, pindolol produces negative chronotropic and inotropic activity. Both of these actions are reversed somewhat, but not entirely, by the drug's partial agonist activity. The negative chronotropic action of pindolol on the sinoatrial node results in a decrease in sinoatrial node discharge and recovery time, thereby decreasing stress- and exercise-stimulated heart rate. Pindolol has a lesser effect on resting heart rate than do beta-adrenergic blocking agents that do not possess intrinsic sympathomimetic activity, usually decreasing resting heart rate only by about 4-8 beats/min or not at all ... Pindolol has a lesser effect on resting cardiac output than on that stimulated by exercise. The decrease in myocardial contractility, blood pressure, and heart rate produced by pindolol during stress and exercise leads to a reduction in myocardial oxygen consumption which accounts for the effectiveness of the drug in chronic stable angina pectoris. Pindolol is probably not effective in patients who develop angina at rest or at low exercise levels.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
Unlike other beta-adrenergic blocking agents, pindolol does not consistently suppress plasma renin activity. In some studies, the drug has increased plasma renin concentrations and reversed the suppression of plasma renin induced by other beta-adrenergic blocking agents. Pindolol has not been shown to cause sodium retention.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1778
For more Mechanism of Action (Complete) data for PINDOLOL (7 total), please visit the HSDB record page.
Related Excipient Companies

Excipients by Applications
Global Sales Information

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
52
PharmaCompass offers a list of Pindolol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pindolol manufacturer or Pindolol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pindolol manufacturer or Pindolol supplier.
PharmaCompass also assists you with knowing the Pindolol API Price utilized in the formulation of products. Pindolol API Price is not always fixed or binding as the Pindolol Price is obtained through a variety of data sources. The Pindolol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pindolol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pindolol, including repackagers and relabelers. The FDA regulates Pindolol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pindolol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pindolol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pindolol supplier is an individual or a company that provides Pindolol active pharmaceutical ingredient (API) or Pindolol finished formulations upon request. The Pindolol suppliers may include Pindolol API manufacturers, exporters, distributors and traders.
click here to find a list of Pindolol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pindolol DMF (Drug Master File) is a document detailing the whole manufacturing process of Pindolol active pharmaceutical ingredient (API) in detail. Different forms of Pindolol DMFs exist exist since differing nations have different regulations, such as Pindolol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pindolol DMF submitted to regulatory agencies in the US is known as a USDMF. Pindolol USDMF includes data on Pindolol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pindolol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pindolol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Pindolol Drug Master File in Japan (Pindolol JDMF) empowers Pindolol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Pindolol JDMF during the approval evaluation for pharmaceutical products. At the time of Pindolol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Pindolol suppliers with JDMF on PharmaCompass.
A Pindolol CEP of the European Pharmacopoeia monograph is often referred to as a Pindolol Certificate of Suitability (COS). The purpose of a Pindolol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Pindolol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Pindolol to their clients by showing that a Pindolol CEP has been issued for it. The manufacturer submits a Pindolol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Pindolol CEP holder for the record. Additionally, the data presented in the Pindolol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Pindolol DMF.
A Pindolol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Pindolol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Pindolol suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pindolol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pindolol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pindolol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pindolol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pindolol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pindolol suppliers with NDC on PharmaCompass.
Pindolol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pindolol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pindolol GMP manufacturer or Pindolol GMP API supplier for your needs.
A Pindolol CoA (Certificate of Analysis) is a formal document that attests to Pindolol's compliance with Pindolol specifications and serves as a tool for batch-level quality control.
Pindolol CoA mostly includes findings from lab analyses of a specific batch. For each Pindolol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pindolol may be tested according to a variety of international standards, such as European Pharmacopoeia (Pindolol EP), Pindolol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pindolol USP).
