
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
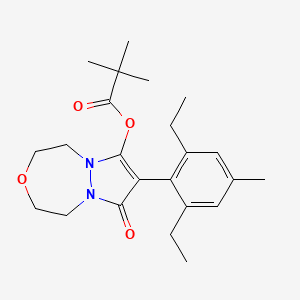

1. 243973-20-8
2. Pinoxaden [iso]
3. U55glf9lv9
4. [8-(2,6-diethyl-4-methylphenyl)-7-oxo-1,2,4,5-tetrahydropyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl] 2,2-dimethylpropanoate
5. Chebi:83524
6. Pinoxaden 100 Microg/ml In Acetonitrile
7. 8-(2,6-diethyl-4-methylphenyl)-1,2,4,5-tetrahydro-7-oxo-7h-pyrazolo(1,2-d)(1,4,5)oxadiazepin-9-yl 2,2-dimethylpropanoate
8. Propanoic Acid, 2,2-dimethyl-, 8-(2,6-diethyl-4-methylphenyl)-1,2,4,5-tetrahydro-7-oxo-7h-pyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl Ester
9. Unii-u55glf9lv9
10. Hsdb 7884
11. [8-(2,6-diethyl-4-methylphenyl)-9-oxo-1,2,4,5-tetrahydropyrazolo[1,2-d][1,4,5]oxadiazepin-7-yl] 2,2-dimethylpropanoate
12. 8-(2,6-diethyl-p-tolyl)-1,2,4,5-tetrahydro-7-oxo-7h-pyrazolo(1,2-d)(1,4,5)oxadiazepin-9-yl 2,2-dimethylpropionate
13. 8-(2,6-diethyl-p-tolyl)-1,2,4,5-tetrahydro-7-oxo-7h-pyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl 2,2-dimethylpropionate
14. Propanoic Acid, 2,2-dimethyl-, 8-(2,6-diethyl-4-methylphenyl)-1,2,4,5-tetrahydro-7-oxo-7h-pyrazolo(1,2-d)(1,4,5)oxadiazepin-9-yl Ester
15. Pinoxaden [mi]
16. Dsstox_cid_14823
17. Dsstox_gsid_34823
18. Schembl64416
19. Chembl1866631
20. Dtxsid8034823
21. Bcp30997
22. Zinc3620800
23. Tox21_304033
24. Ncgc00163860-01
25. Ncgc00356993-01
26. 8-(2,6-diethyl-4-methylphenyl)-7-oxo-1,2,4,5-tetrahydro-7h-pyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl 2,2-dimethylpropanoate
27. Cas-243973-20-8
28. Ft-0778659
29. Pinoxaden, Pestanal(r), Analytical Standard
30. A845290
31. Q15632855
32. [8-(2,6-diethyl-4-methyl-phenyl)-7-oxidanylidene-1,2,4,5-tetrahydropyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl] 2,2-dimethylpropanoate
33. 2,2-dimethylpropanoic Acid [8-(2,6-diethyl-4-methylphenyl)-7-oxo-1,2,4,5-tetrahydropyrazolo[1,2-d][1,4,5]oxadiazepin-9-yl] Ester
34. Propanoic Acid, 2,2-dimethyl-, 8-(2,6-diethyl-4-methylphenyl)-1,2,4,5-tetrahydro-7-oxo-7h-pyrazolo1,2-d1,4,5oxadiazepin-9-yl Ester
| Molecular Weight | 400.5 g/mol |
|---|---|
| Molecular Formula | C23H32N2O4 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 400.23620751 g/mol |
| Monoisotopic Mass | 400.23620751 g/mol |
| Topological Polar Surface Area | 59.1 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 652 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
In a ... rabbit metabolism study, a single oral dose of (Phenyl-1-(14)C) NOA 407855 (/pinoxaden/ Batch/lot # ILA-8.1B-5 and ILA-8.1C-1B; radiochemical purity $98.9%) was administered in aqueous 0.5% carboxymethylcellulose and 0.1% Tween 80 to female Chbb-HM rabbits (3/dose) via gavage at nominal dose levels of 0.5 and 300 mg/kg. Urine, feces, and blood samples were collected up to 168 hours after dosing. Metabolites in urine and feces were quantified and identified by HPLC, TLC, and LC/MS. Animals were sacrificed after 168 hours, and tissues were collected to determine of residual radioactivity. Absorption and elimination of (14)C NOA 407855 were rapid and essentially complete regardless of dose level. Maximum concentrations (Cmax) in blood were attained within 0.5 hours at the low dose and within approximately 2 hours at the high dose. Half lives for radioactivity in the blood were 3 and 12 hours for the low and high dose groups, and radioactivity in blood was non-detectable by 48 and 96 hours for the low and high dose groups. The total recovery of the radioactive dose averaged 94.7-100.1% at 168 hours post-dose. The route of excretion was essentially independent of dose, although excretion was slightly retarded at the high dose compared to the low dose. Approximately 90% of the dose was excreted in the urine within 24 (low dose) or 48 (high dose) hours. An additional 4-7% dose was excreted in the feces within 48 hours. For both dose groups, concentrations of radioactivity remaining in the tissues were negligible by 168 hours post-dose and accounted for less than or equal to 0.1% of the dose. Quantifiable residues were detect in gall bladder and gastrointestinal (GI) tract for both the low dose group (0.0020-0.0025 ppm Eq) and high dose group (1.66-1.69 ppm Eq), and in the kidneys, liver, plasma, and blood (0.017-0.158 ppm) of the high dose group. However, radioactivity in the remaining tissues was below the limit of quantitation (LOQ). Essentially all of the metabolites excreted in the urine and feces were identified (92.8-97.7% dose), and the metabolite profile was the same regardless of dose level. For both dose groups, Metabolite M2 (NOA 407854) was identified as the major component in both urine (88.6-91.0% dose) and feces (3.6-6.2% dose). Minor amounts of Metabolites M4 (0.4-0.6% dose), K4 (0.2% dose), M12 (0.2% dose) and K3 (0.1% dose) were also identified in urine and/or feces. Unidentified metabolites accounted for less than or equal to 0.6% dose. In rabbits, the metabolism of NOA 407885 proceeds predominantly by hydrolysis of the ester linkage to form Metabolite M2 (NOA 407854), which is the major metabolite in urine and feces (92-97% dose). Minor secondary reactions include either: hydroxylation at the 4-methyl group of the phenyl moiety to yield M4 (excreted in urine and feces); or glucuronidation of M2 to form metabolite M12. The metabolic pathway in the rabbit is essentially identical to the rat.
USEPA; Pinoxaden: Human Health Risk Assessment.p.76-7 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
Dermal absorption is estimated to be 40% based on the results of the in vivo/in vitro dermal penetration study in rats using the EC 100 formulation. (The emulsifiable concentrate (EC) formulation will be used in the field and is believed to be much more absorbable than technical pinoxaden without the emulsifiers.) In this study with the EC formulation, 36% of the dose applied to the skin of rats in an in vivo study was absorbed over the following day (24 hours post-exposure). Absorption of the EC formulation from excised rat skin in an in vitro study was 65.5% of the applied dose after 24 hours post-exposure. For excised human skin, absorption of radioactivity was minimal regardless of the dosing vehicle and dose level in an in vitro study. Absorption accounted 0.36-1.84% of the applied dose after a 24-hour exposure at doses from 5-400 :g/cm2. Thus, in the in vitro studies, absorption was considerably higher in rat skin than in human skin. Additionally, absorption of the test substance in the in vivo rat study was comparable to the absorption in the in vitro study with rat skin. Therefore, the data suggest that in vivo absorption in humans would be considerably lower than in the rat.
USEPA; Pinoxaden: Human Health Risk Assessment.p.39 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
In a ... mouse metabolism study, (pyrazol-3,5-(14)C) NOA 407855 (/pinoxaden/ greater than or equal to 97.6% radiochemical purity; Lots ILA-76.3B ILA-76.3C) was administered to 4 groups of male and female C57BL/10Jf/CD-1 mice as follows: (I) Group G1 was given either a single-dose or repeated daily dose of 1.4 mg/kg body-weight by gavage; (ii) Group G2 was administered either a single-dose or repeated daily dose of 140 mg/kg body-weight by gavage; (iii) Group G3 was fed at 10 ppm in the diet; and (iv) Group G4 was fed a 1000 ppm diet. The maximum duration of dosing in each group was for 18 days. Terminal blood samples were collected to determine a time course of the concentration of radioactivity in whole blood, and urine and feces were collected over 24 hours after varying time periods of dosing. The stated objectives of this study were to: (I) investigate the duration of dosing required to reach steady-state kinetics; (ii) compare systemic exposure following gavage or dietary dosing and determine any marked sex difference; (iii) compare blood and excreta metabolite profiles after single and multiple dosing to male and female mice; and (iv) resolve which metabolite should be analyzed during dietary studies. Radioactivity levels in whole blood sampled at various post-dose intervals indicated that the gavage dose was rapidly absorbed (Tmax = 0.5 hours) and eliminated regardless of the dose level or the duration of dosing. Absorption was slower for the dietary dosing groups (Tmax = 8-12 hours), but elimination from the blood was rapid once mice were withdrawn from the treated diet. Concentrations in blood at Tmax following 1, 7, 14, and 18 days of dietary dosing indicated that a steady state in blood levels was achieved within 18 days. Although urinary excretion was typically higher than fecal excretion, there was no clear pattern in the distribution of excreted radioactivity between urine and feces based on sex, dosing group, or duration of dosing. Radioactivity in urine (including cage wash) varied from 26-83% of the excreted radioactivity and in feces from 17-74%. Following oral administration of NOA 407855 to mice either by gavage or in the diet, the metabolite profiles in blood, urine and feces were qualitatively and quantitatively independent of sex, dose level, dosing method (gavage vs. dietary), dosing duration (single vs. multiple doses) and time of collection, although some quantitative variations were observed in feces. Parent compound was not detected in blood, urine or feces. Metabolite M2 (NOA 407854) was the major component identified in all three matrices, accounting for approximately 67-93% of the extractable blood radioactivity, 69-89% of the total radioactivity in urine, and 35-75% of the radioactivity extractable from feces. Substantial amounts of Metabolite M4 were also detected in blood (2-11% extractable blood radioactivity), urine (5-14% of total radioactivity), and feces (12-41% of the extractable radioactivity). The remaining components detected in each matrix were minor (<8% of the sample radioactivity) and included five components in blood extracts, eight components in urine, and 8-11 components in fecal extracts. The presence of Metabolites M2 and M4 in urine and feces were confirmed by LC/MS and LC/NMR analyses of fractions isolated from composited samples. These analyses also identified minor amounts (<3% sample radioactivity) of Metabolites M13, M21, M50, and M51 in urine and Metabolites M13, M19, M20, M22, M49, and M50 in fecal extracts. Based on the metabolites identified in blood, urine and feces and their relative abundance, the metabolism of NOA 407855 in mice primarily involves hydrolysis of the ester moiety to form Metabolite M2, which is the primary component excreted in urine and feces. To a minor extent, Metabolite M2 may also undergo a number of secondary reactions to produce variety of minor metabolites. These secondary reactions include: hydroxylation, oxidation, hydrolysis, dealkylation, ring formation, and cleavage of the ether bond in the oxadiazepine moiety.
USEPA; Pinoxaden: Human Health Risk Assessment.p.77-8 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
In an in vivo dermal penetration study, (pyrazole-3, 5-(14)C) NOA407855 (/pinoxaden/ >95% radiochemical purity, lot/batch #EZ005006) was suspended in an emulsifiable concentrate (EC) formulation and applied neat or as aqueous dilutions to approximate exposure to the undiluted commercial formulation and to the dilute aqueous spray used in the field. The formulated test substance was administered to the shaved intact skin (10 sq cm) of 4 male Alpk:APfSD (Wistar-derived) rats/time point/dose at dose levels of 5 and 25 ug/sq cm for the aqueous dilutions (1/200 and 1/40) and 400 ug/cm2 for the neat EC formulation for a 4- or 10-hour exposure period. At the end of the exposure period, the skin of each rat was washed, and 4 rats/time point/dose were sacrificed for examination of dermal-absorption. A further 4 rats/dose were exposed for 10 hours and then retained for 24 hours after the skin was washed to determine further post-exposure absorption. In addition, in vitro studies were conducted using excised rat skin and human skin mounted in a static diffusion cell apparatus to compare dermal-absorption. The dosing regimen used in the in vitro studies was the same as in the in vivo study, except that an additional dose level of 1000 ug/sq cm was employed for human skin using the neat EC formulation. Absorption from excised skin samples was examined after 10- and 24-hour exposure durations, except at the 1000 ug/sq cm dose level which used only a 10-hour exposure. For the in vivo rat study, total recovery of the applied dose ranged from 84-96% for all dose groups. For the neat EC formulation (400 ug/sq cm), 17% of the dose was absorbed after 4 hours and 30% dose after 10 hours, increasing to 36% dose over the following day (24 hours post-exposure). Regardless of exposure duration, 7% dose remained available in or on the skin for potential absorption, with 1.3-1.4% of the dose in the stratum corneum. At 24 hours post-exposure, the potentially absorbable dose declined to 5.3% dose, with 1.4% of the dose in the stratum corneum. Most of the absorbed radioactivity was excreted in the urine (including cage wash), accounting for approximately 30% of the applied dose (83% of the absorbed dose), and 3.3% dose was eliminated in the feces (9% of the absorbed dose). Excretion was virtually complete within 24 hours, with only 1.5% of the dose being recovered in the GI tract and carcass. For the 1/40 aqueous spray dilution (25 ug/sq cm), absorption was markedly lower than for the EC formulation, with only 0.7 and 1.6% of the dose being absorbed by 4 and 10 hours, respectively. After a 10-hour exposure and washing, there was an increase in absorption up to 3.8% dose by 24 hours post-dose. Potentially absorbable radioactivity in or on the skin following washing accounted for 2.6-3.1% of the dose at all sampling intervals and was primarily associated with the stratum corneum (2.0-2.3% dose). As with the high-dose group, most of the absorbed radioactivity was excreted within 24 hours in the urine (66% of absorbed dose) and feces (11% of absorbed dose). For the in vitro studies using excised rat and human skin, total recovery of the applied dose ranged from 94-104% for all dose groups at both exposure intervals. As in the in vivo study, absorption from excised rat skin was higher for the neat EC formulation (400 ug/sq cm) than for either of the 1/200 or 1/40 aqueous dilutions (5 and 25 ug/sq cm, respectively). Following a 10-hour exposure, 40.3% of the applied dose from the EC formulation was absorbed compared to 34.1 and 25.0% of the applied dose from aqueous dilutions (5 and 25 ug/sq cm). After 24 hours of exposure, absorption rose to 65.5% dose for the EC formulation and 49.0 and 44.7% dose for the aqueous dilutions. Regardless of exposure duration, potentially absorbable radioactivity remaining on the skin accounted for 8.8-11.8% dose for the neat EC formulation, 12.1-12.9% dose for the 1/40 aqueous dilution, and 18.5-20.6% dose for the 1/200 aqueous dilution. For excised human skin, absorption of radioactivity was minimal regardless of the dosing vehicle and dose level. Absorption accounted for 0.34-1.55% of the applied dose after a 10-hour exposure at doses from 5-1000 ug/sq cm and 0.36-1.84% of the applied dose after a 24-hour exposure at doses from 5-400 ug/sq cm. Potentially absorbable radioactivity remaining in or on the skin (stratum corneum and epidermis) accounted for 2.42-3.61% dose in the greater than or equal to 25 ug/sq cm dose groups and 8.49-8.80% dose in the 5 ug/sq cm dose group. Thus, in the in vitro studies, absorption was considerably higher in rat skin than in human skin. Additionally, absorption of the test substance in the in vivo rat study was comparable to the absorption in the in vitro study with rat skin. Therefore, the data may suggest that in vivo absorption in humans would be considerably lower than in the rat.
USEPA; Pinoxaden: Human Health Risk Assessment.p.80-1 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
From livestock metabolism studies, the residues with concentrations >10% total radioactive residue were chosen as residues of concern for livestock. Therefore, besides the parent compound, M2 and M4 (free and conjugated) for ruminants and M2, M4 (free and conjugated), and M6 for poultry were determined to be residues of concern. M4 is the major metabolite in the livestock. ((14)Cphenyl)-pinoxaden and ((14)C-phenyl)-M4 were the only compounds that were fed to ruminants in two separate studies.
USEPA; Pinoxaden: Human Health Risk Assessment.p.20 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
The metabolism of pinoxaden in rats primarily involves the initial hydrolysis of the ester moiety to form metabolite M2 (NOA 407854), which is then extensively excreted in the urine and feces. To a minor extent, metabolite M2 is also further metabolized via hydroxylation, dealkylation, ring cleavage, ring formation, and conjugation into a wide variety of minor metabolites. The proposed pathway is also supported by the appended in vitro study, which indicates that pinoxaden is rapidly hydrolyzed to M2 in rat plasma (half life = approximately 0.1 min) at concentrations up to 100 uM (approximately 40 ppm).
USEPA; Pinoxaden: Human Health Risk Assessment.p.14 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
In a ... rabbit metabolism study, a single oral dose of (Phenyl-1-(14)C) NOA 407855 (/pinoxaden/ Batch/lot # ILA-8.1B-5 and ILA-8.1C-1B; radiochemical purity $98.9%) was administered in aqueous 0.5% carboxymethylcellulose and 0.1% Tween 80 to female Chbb-HM rabbits (3/dose) via gavage at nominal dose levels of 0.5 and 300 mg/kg. Urine, feces, and blood samples were collected up to 168 hours after dosing. Metabolites in urine and feces were quantified and identified by HPLC, TLC, and LC/MS. ... Essentially all of the metabolites excreted in the urine and feces were identified (92.8-97.7% dose), and the metabolite profile was the same regardless of dose level. For both dose groups, Metabolite M2 (NOA 407854) was identified as the major component in both urine (88.6-91.0% dose) and feces (3.6-6.2% dose). Minor amounts of Metabolites M4 (0.4-0.6% dose), K4 (0.2% dose), M12 (0.2% dose) and K3 (0.1% dose) were also identified in urine and/or feces. Unidentified metabolites accounted for less than or equal to 0.6% dose. In rabbits, the metabolism of NOA 407885 proceeds predominantly by hydrolysis of the ester linkage to form Metabolite M2 (NOA 407854), which is the major metabolite in urine and feces (92-97% dose). Minor secondary reactions include either: hydroxylation at the 4-methyl group of the phenyl moiety to yield M4 (excreted in urine and feces); or glucuronidation of M2 to form metabolite M12. The metabolic pathway in the rabbit is essentially identical to the rat.
USEPA; Pinoxaden: Human Health Risk Assessment.p.76-7 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
In a ... mouse metabolism study, (pyrazol-3,5-(14)C) NOA 407855 (/pinoxaden/ greater than or equal to 97.6% radiochemical purity; Lots ILA-76.3B ILA-76.3C) was administered to 4 groups of male and female C57BL/10Jf/CD-1 mice as follows: (I) Group G1 was given either a single-dose or repeated daily dose of 1.4 mg/kg body-weight by gavage; (ii) Group G2 was administered either a single-dose or repeated daily dose of 140 mg/kg body-weight by gavage; (iii) Group G3 was fed at 10 ppm in the diet; and (iv) Group G4 was fed a 1000 ppm diet. The maximum duration of dosing in each group was for 18 days. ... Following oral administration of NOA 407855 to mice either by gavage or in the diet, the metabolite profiles in blood, urine and feces were qualitatively and quantitatively independent of sex, dose level, dosing method (gavage vs. dietary), dosing duration (single vs. multiple doses) and time of collection, although some quantitative variations were observed in feces. Parent compound was not detected in blood, urine or feces. Metabolite M2 (NOA 407854) was the major component identified in all three matrices, accounting for approximately 67-93% of the extractable blood radioactivity, 69-89% of the total radioactivity in urine, and 35-75% of the radioactivity extractable from feces. Substantial amounts of Metabolite M4 were also detected in blood (2-11% extractable blood radioactivity), urine (5-14% of total radioactivity), and feces (12-41% of the extractable radioactivity). The remaining components detected in each matrix were minor (<8% of the sample radioactivity) and included five components in blood extracts, eight components in urine, and 8-11 components in fecal extracts. The presence of Metabolites M2 and M4 in urine and feces were confirmed by LC/MS and LC/NMR analyses of fractions isolated from composited samples. These analyses also identified minor amounts (<3% sample radioactivity) of Metabolites M13, M21, M50, and M51 in urine and Metabolites M13, M19, M20, M22, M49, and M50 in fecal extracts. Based on the metabolites identified in blood, urine and feces and their relative abundance, the metabolism of NOA 407855 in mice primarily involves hydrolysis of the ester moiety to form Metabolite M2, which is the primary component excreted in urine and feces. To a minor extent, Metabolite M2 may also undergo a number of secondary reactions to produce variety of minor metabolites. These secondary reactions include: hydroxylation, oxidation, hydrolysis, dealkylation, ring formation, and cleavage of the ether bond in the oxadiazepine moiety.
USEPA; Pinoxaden: Human Health Risk Assessment.p.77-8 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
In a ... study designed to correlate the levels of Metabolite M2 (NOA 407854) in the blood with the ingestion of NOA 407855 /pinoxaden/ in the diet, four groups of C57Bl/10JfCD-1 mice (24/sex/dose group) were fed diets containing NOA 407855 (97.2% ai, Lot #EZ005006) for at least 39 consecutive days. Two groups were fed diets containing NOA 407855 at 1000 or 2500 ppm for the entire period; the third group was fed at 2500 ppm for 7 days, followed by 5000 ppm for at least 32 consecutive days; and the fourth group was fed at 2500 ppm for 7 days, followed by 5000 ppm for 14 days, and then 7000 ppm for at least 18 days. A fifth group of mice (3 or 4/sex) of the same source and strain served as controls for the duration of the study. ... Concentrations of M2 in blood on Study days 40 and 41 averaged 1.7-2.3 mg/kg at 1000 mg/kg, 4.6-7.4 mg/kg at 2500 ppm, 11.8-12.7 mg/kg at 5000 ppm, and 17.0-20.8 mg/kg at 7000 ppm. Linear regression showed a direct correlation between the concentration of M2 in the blood and the concentration of the test material in the diet, with R2 = 0.96 for females and 0.98 for males. There were no apparent differences in M2 blood concentrations between sexes or time of blood sampling at any dose.
USEPA; Pinoxaden: Human Health Risk Assessment.p.78-9 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
In a ... rabbit metabolism study, a single oral dose of (Phenyl-1-(14)C) NOA 407855 (/pinoxaden/ Batch/lot # ILA-8.1B-5 and ILA-8.1C-1B; radiochemical purity $98.9%) was administered in aqueous 0.5% carboxymethylcellulose and 0.1% Tween 80 to female Chbb-HM rabbits (3/dose) via gavage at nominal dose levels of 0.5 and 300 mg/kg. Urine, feces, and blood samples were collected up to 168 hours after dosing. ... Half lives for radioactivity in the blood were 3 and 12 hours for the low and high dose groups, and radioactivity in blood was non-detectable by 48 and 96 hours for the low and high dose groups.
USEPA; Pinoxaden: Human Health Risk Assessment.p.76 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf
Pinoxaden (NOA 407855) is a representative of the new phenylpyrazolin class of chemicals. The mode of action is the inhibition of the enzyme, acetyl-coenzyme A carboxylase (ACCase). ACCase activity in plants can be attributed to two isoenzymes located in different compartments of the plant cell, the chloroplast and the cytosol. The chloroplastic enzyme is responsible for the de novo biosynthesis of all fatty acids in the cell. The malonyl-coenzyme A produced by the cytosolic ACCase is required for fatty acid elongation to form very long-chain fatty acids, and for the biosynthesis of flavonoids and malonylated metabolites. Pinoxaden has been found to inhibit both the chloroplastic and the cytosolic ACCase enzyme in gramineae.
USEPA; Pinoxaden: Human Health Risk Assessment.p.5 (July 13, 2005). Available from, as of February 10, 2011: https://www.epa.gov/opprd001/factsheets/humanhealth_pinoxaden.pdf

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
19
PharmaCompass offers a list of Pinoxaden API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pinoxaden manufacturer or Pinoxaden supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pinoxaden manufacturer or Pinoxaden supplier.
PharmaCompass also assists you with knowing the Pinoxaden API Price utilized in the formulation of products. Pinoxaden API Price is not always fixed or binding as the Pinoxaden Price is obtained through a variety of data sources. The Pinoxaden Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pinoxaden manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pinoxaden, including repackagers and relabelers. The FDA regulates Pinoxaden manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pinoxaden API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pinoxaden manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pinoxaden supplier is an individual or a company that provides Pinoxaden active pharmaceutical ingredient (API) or Pinoxaden finished formulations upon request. The Pinoxaden suppliers may include Pinoxaden API manufacturers, exporters, distributors and traders.
click here to find a list of Pinoxaden suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Pinoxaden Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pinoxaden GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pinoxaden GMP manufacturer or Pinoxaden GMP API supplier for your needs.
A Pinoxaden CoA (Certificate of Analysis) is a formal document that attests to Pinoxaden's compliance with Pinoxaden specifications and serves as a tool for batch-level quality control.
Pinoxaden CoA mostly includes findings from lab analyses of a specific batch. For each Pinoxaden CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pinoxaden may be tested according to a variety of international standards, such as European Pharmacopoeia (Pinoxaden EP), Pinoxaden JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pinoxaden USP).
