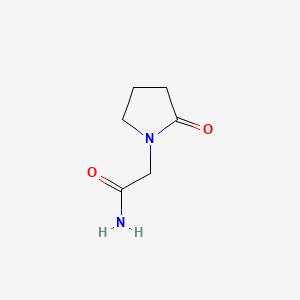

1. 2-pyrrolidone-n-acetamide
2. Avigilen
3. Axonyl
4. Cerebroforte
5. Cerepar N
6. Ciclofalina
7. Cuxabrain
8. Dinagen
9. Gabacet
10. Geram
11. Memo Puren
12. Memo-puren
13. Nootrop
14. Nootropil
15. Nootropyl
16. Normabran
17. Piracebral
18. Piracetam Abz
19. Piracetam Rph
20. Piracetam-rph
21. Piracetrop
22. Pirazetam
23. Pyracetam
24. Pyramem
25. Sinapsan
26. Ucb 6215
27. Ucb-6215
28. Ucb6215
1. 7491-74-9
2. 2-(2-oxopyrrolidin-1-yl)acetamide
3. Nootropil
4. 2-oxo-1-pyrrolidineacetamide
5. Nootropyl
6. Pyracetam
7. 1-pyrrolidineacetamide, 2-oxo-
8. Normabrain
9. Gabacet
10. Pyramem
11. 2-pyrrolidinoneacetamide
12. Genogris
13. Pirroxil
14. Euvifor
15. Nootron
16. Myocalm
17. 2-oxo-pyrrolidine Acetamide
18. 2-pyrrolidoneacetamide
19. Cl-871
20. Ucb 6215
21. Ucb-6215
22. 2-(2-oxo-pyrrolidin-1-yl)acetamide
23. 2-oxo-pyrrolidin-1-ylacetamide
24. 2-ketopyrrolidine-1-ylacetamide
25. 2-(2-oxopyrrolidino)acetamide
26. Mls000069719
27. 2-(2-oxo-1-pyrrolidinyl)acetamide
28. Ciclofalina
29. Nsc-758191
30. Smr000058196
31. Zh516lnz10
32. Pirazetam
33. Ncgc00015821-02
34. Cas-7491-74-9
35. Dsstox_cid_24491
36. Dsstox_rid_80267
37. Dsstox_gsid_44491
38. Naofukang [chinese]
39. Cerebroforte
40. Piracetamum
41. Avigilen
42. Breinox
43. Naofukang
44. Nootrop
45. Norzetam
46. Axonyl
47. Geram
48. Piracetamum [inn-latin]
49. Kt-801
50. Sr-01000076071
51. Einecs 231-312-7
52. Brn 1526393
53. Unii-zh516lnz10
54. Encetrop
55. Piracetam [usan:inn:ban]
56. Hsdb 7529
57. Piracetam,(s)
58. Prestwick_870
59. Myocalm (tn)
60. Deshydroxy Oxiracetam
61. Spectrum_001421
62. Piracetam [inn]
63. Piracetam [jan]
64. Piracetam [mi]
65. Piracetam [hsdb]
66. Piracetam [inci]
67. Piracetam [usan]
68. Opera_id_1766
69. Prestwick0_000537
70. Prestwick1_000537
71. Prestwick2_000537
72. Prestwick3_000537
73. Spectrum2_001074
74. Spectrum3_001523
75. Spectrum4_000742
76. Spectrum5_001037
77. Lopac-p-5295
78. Piracetam [mart.]
79. Cid_4843
80. Piracetam [who-dd]
81. Lopac0_000949
82. Oprea1_512927
83. Schembl20172
84. Bspbio_000553
85. Bspbio_002906
86. Kbiogr_001064
87. Kbioss_001901
88. 5-21-06-00360 (beilstein Handbook Reference)
89. Piracetam (jan/usan/inn)
90. 2-oxo-1-pyrrolidinylacetamide
91. Chembl36715
92. Divk1c_000259
93. Spectrum1502195
94. Spbio_001088
95. Spbio_002474
96. 2-(2-ketopyrrolidino)acetamide
97. Bpbio1_000609
98. Gtpl4288
99. Dtxsid5044491
100. Piracetam [ep Monograph]
101. Bdbm62877
102. Chebi:32010
103. Cl-781
104. Hms500m21
105. Kbio1_000259
106. Kbio2_001901
107. Kbio2_004469
108. Kbio2_007037
109. Kbio3_002406
110. Ninds_000259
111. Hms1569l15
112. Hms1921l12
113. Hms2092d18
114. Hms2096l15
115. Hms2230b24
116. Hms3262n20
117. Hms3371g01
118. Hms3657a05
119. Hms3713l15
120. Hms3885i07
121. Pharmakon1600-01502195
122. Bcp28414
123. Hy-b0585
124. Zinc3812874
125. Tox21_110229
126. Tox21_301990
127. Tox21_500949
128. Bbl028161
129. Ccg-39282
130. Nsc758191
131. S3070
132. Stk535612
133. 2 - Oxo - 1 - Pyrrolidineacetamide
134. Akos001038683
135. Tox21_110229_1
136. 2-(2-oxo-1-pyrrolidinyl)acetamide #
137. Db09210
138. Lp00949
139. Nsc 758191
140. Sdccgsbi-0050923.p004
141. Idi1_000259
142. Ncgc00015821-01
143. Ncgc00015821-03
144. Ncgc00015821-04
145. Ncgc00015821-05
146. Ncgc00015821-06
147. Ncgc00015821-07
148. Ncgc00015821-08
149. Ncgc00015821-10
150. Ncgc00015821-21
151. Ncgc00094253-01
152. Ncgc00094253-02
153. Ncgc00094253-03
154. Ncgc00094253-04
155. Ncgc00255727-01
156. Ncgc00261634-01
157. Ac-33158
158. As-13920
159. Bp166248
160. Sbi-0050923.p003
161. Db-019407
162. Ab00052287
163. Eu-0100949
164. Ft-0636504
165. P2880
166. Sw196995-3
167. 2-(2-oxidanylidenepyrrolidin-1-yl)ethanamide
168. Piracetam, Vetranal(tm), Analytical Standard
169. D01914
170. P 5295
171. Ab00052287_12
172. A838261
173. Ae-641/30117005
174. Q410069
175. Sr-01000076071-1
176. Sr-01000076071-4
177. Sr-01000076071-6
178. W-104408
179. Brd-k19456237-001-22-7
180. Z56865289
181. Piracetam, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 142.16 g/mol |
|---|---|
| Molecular Formula | C6H10N2O2 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 142.074227566 g/mol |
| Monoisotopic Mass | 142.074227566 g/mol |
| Topological Polar Surface Area | 63.4 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 167 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/Investigators/ report on a 30-year-old patient with advanced cerebellar degeneration due to sickle cell amemia 2. He presented with severe myoclonus, which was resistant to conventional therapy and dramatically improved after administration of 12-18 g/day piracetam. Piracetam may be considered in the treatment of refractory myoclonus in spinocerebellar degenerations.
PMID:16149096 De Rosa A et al; Mov Disord 21 (1): 116-8 (2006)
/Piracetam/ is indicated for patients suffering from myoclonus of cortical origin, irrespective of etiology, and should be used in combination with other anti-myoclonic therapies.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Piracetam is contraindicated in patients with severe renal impairment (renal creatinine clearance of less than 20 mL per minute), hepatic impairment and to those under 16 years of age.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Piracetam is contraindicated in patients with cerebral hemorrhage and in those with hypersensitivity to piracetam, other pyrrolidone derivatives or any of the excipients.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Due to the effect of piracetam on platelet aggregation, caution is recommended in patients with underlying disorders of hemostasis, major surgery or severe hemorrhage.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Abrupt discontinuation of treatment should be avoided as this may induce myoclonic or generalised seizures in some myoclonic patients.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
For more Drug Warnings (Complete) data for PIRACETAM (9 total), please visit the HSDB record page.
Indicated in adult patients suffering from myoclonus of cortical origin, irrespective of aetiology, and should be used in combination with other anti-myoclonic therapies.
Piracetam is known to mediate various pharmacodynamic actions: **Neuronal effects**: Piracetam modulates the cholinergic, serotonergic, noradrenergic, and glutamatergic neurotransmission although the drug does not display high affinity to any of the associated receptors (Ki >10M). Instead, piracetam increases the density of postsynaptic receptors and/or restore the function of these receptors through stabilizing the membrane fluidity. In the forebrain of aging mice, the density of NMDA receptors was increased by approximately 20% following 14 days of piracetam treatment. Based on the findings of various animal and human studies, the cognitive processses including learning, memory, attention and consciousness were enhanced from piracetam therapy without inducing sedation and psychostimulant effects. Piracetam mediate neuroprotective effects against hypoxia-induced damage, intoxication, and electroconvulsive therapy. In two studies involving alcohol-treated rats with evidences of withdrawal-related neuronal loss, piracetam was shown to reduce the extent of neuronal loss and increase the numbers of synapses in the hippocampus by up to 20% relative to alcohol-treated or alcohol-withdrawn rats. This suggests that piracetam is capable in promoting neuroplasticity when recoverable neural circuits are present. Although the mechanism of action is not fully understood, administration of piracetam prior to a convulsant stimulus reduces the seizure severity and enhances the anticonvulsant effectiveness of conventional antiepileptics such as carbamazepine and diazepam. **Vascular effects**: Piracetam is shown to increase the deformability of erythrocytes, reduce platelet aggregation in a dose-dependent manner, reduce the adhesion of erythrocytes to vascular endothelium and capillary vasospasm. In healthy volunteers, piracetam mediated a direct stimulant effect on prostacycline synthesis and reduced the plasma levels of fibrinogen and von Willebrands factors (VIII: C; VIII R: AG; VIII R: vW) by 30 to 40%. Potentiated microcirculation is thought to arise from a combination of effects on erythrocytes, blood vessels and blood coagulation.
Nootropic Agents
Drugs used to specifically facilitate learning or memory, particularly to prevent the cognitive deficits associated with dementias. These drugs act by a variety of mechanisms. (See all compounds classified as Nootropic Agents.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
N06BX03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BX - Other psychostimulants and nootropics
N06BX03 - Piracetam
Absorption
Piracetam displays a linear and time-dependent pharmacokinetic properties with low intersubject variability over a large range of doses. Piracetam is rapidly and extensively absorbed following oral administration with the peak plasma concentration is reached within 1 hour after dosing in fasted subjects. Following a single oral dose of 3.2 g piracetam, the peak plasma concentration (Cmax) was 84 g/mL. Intake of food may decrease the Cmax by 17% and increase the time to reach Cmax (Tmax) from 1 to 1.5 hours. Tmax in the cerebrospinal fluid is achieved approximately 5 hours post-administration. The absolute bioavailability of piracetam oral formulations is close to 100% and the steady state plasma concentrations are achieved within 3 days of dosing.
Route of Elimination
Piracetam is predominantly excreted via renal elimination, where about 80-100% of the total dose is recovered in the urine. Approximately 90% of the dose of piracetam is excreted in the urine as unchanged drug.
Volume of Distribution
Vd is approximately 0.6L/kg. Piracetam may cross the blood-brain barrier as it was measured in the cerebrospinal fluid following intravenous administration. Piracetam diffuses to all tissues except adipose tissues, crosses placental barrier and penetrates the membranes of isolated red blood cells.
Clearance
The apparent total body clearance is 80-90 mL/min.
Piracetam is rapidly and almost completely absorbed. Peak plasma levels are reached within 1.5 hours after administration. The extent of oral bioavailability, assessed from the Area Under Curve (AUC), is close to 100% for capsules, tablets and solution.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Peak levels and AUC are proportional to the dose given. The volume of distribution of piracetam is 0.7 L/kg, and ... Clearance of the compound is dependent on the renal creatinine clearance and would be expected to diminish with renal insufficiency.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Piracetam is excreted in human breast milk.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Piracetam crosses the blood-brain and the placental barrier and diffuses across membranes used in renal dialysis.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Piracetam is excreted almost completely in urine and the fraction of the dose excreted in urine is independent of the dose given.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
As large proportion of total piracetam administered is excreted as unchanged drug, there is no known major metabolism of piracetam.
... No metabolite of piracetam has been found.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
The plasma half life of piracetam is approximately 5 hours following oral or intravenous administration. The half life in the cerebrospinal fluid was 8.5 hours.
... The plasma half-life is 5.0 hours, in young adult men.
Medicines.org.uk; Nootropil 800mg & 1200mg Tablets and Solution. (UCB Pharma Limited; August 2005) electronic Medicines Compendium. Datapharm Communications. Available from, as of July 31, 2007: https://emc.medicines.org.uk/
Piracetam interacts with the polar heads in the phospholipids membrane and the resulting mobile drug-lipid complexes are thought to reorganize the lipids and influence membrane function and fluidity. Such interaction has been reported in a study that investigated the effects of neuronal outgrowth induced by beta amyloid peptides; while amyloid peptides cause lipid disorganization within the cell membranes leading to neuronal death, piracetam demonstrated to decrease the destabilizing effects of amyloid peptide. The authors suggest that piracetam induces a positive curvature of the membrane by occupying the polar groups in the phospholipids to counteract the negative curvature induced by amyloid peptides , which in turn would decrease the likelihood of membrane fusion. This mechanism of action is thought to improve membrane stability, allowing the membrane and transmembrane proteins to maintain and recover the three-dimensional structure or folding for normal function such as membrane transport, chemical secretion, and receptor binding and stimulation. Through restored membrane fluidity, piracetam promotes restored neurotransmission such as glutamatergic and cholinergic systems, enhances neuroplasticity and mediates neuroprotective and anticonvulsant effects at the neuronal level. It is also demonstrated that piracetam also improves the fluidity of platelet membranes. At the vascular level, piracetam decreases adhesion of erythrocytes to cell wall and reduces vasospasm which in turn improves microcirculation including cerebral and renal blood flow.
It was found that a drug of the nootropic nature piracetam possessing pronounced antihypoxic properties eliminates calcium chloride-induced disturbances of the cardiac rhythm and significantly raises the threshold of atrial fibrillation during electrical stimulation. The drug's antiarrhythmic effect is followed by a decrease of the rhythm rate and an increase of the contraction amplitude. The animals treated with piracetam in a dose when its antiarrhythmic effects (300 mg/kg) exhibited a decrease of the membrane potential of erythrocytes as compared with control. Similar effects occurred in the animals treated with lidocaine. It can be concluded that in certain types of arrhythmias the use of piracetam restores the normal rhythm of contractions that is perhaps connected with its positive influence on metabolic processes in the myocardium.
PMID:2081561 Samvelian V et al; Farmakol Toksikol 53 (6): 22-3 (1990)