
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Et 495
2. Et-495
3. Et495
4. Eu 4200
5. Eu-4200
6. Eu4200
7. Hydrochloride, Piribedil
8. Mesylate, Piribedil
9. Mono-hydrochloride, Piribedil
10. Piribedil Hydrochloride
11. Piribedil Mesylate
12. Piribedil Mono Hydrochloride
13. Piribedil Mono-hydrochloride
14. Piribendyl
15. Trivastal
1. 3605-01-4
2. Trivastal
3. Trivastan
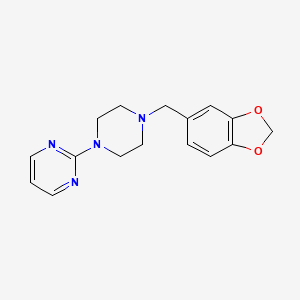
4. 2-(4-(benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)pyrimidine
5. Et-495
6. Eu-4200
7. Et 495
8. 2-(4-piperonyl-1-piperazinyl)pyrimidine
9. 2-[4-(1,3-benzodioxol-5-ylmethyl)piperazin-1-yl]pyrimidine
10. Eu 4200
11. Do22k1prdj
12. 1-(2-pyrimidyl)-4-piperonylpiperazine
13. Pyrimidine, 2-(4-piperonyl-1-piperazinyl)-
14. Pyrimidine, 2-[4-(1,3-benzodioxol-5-ylmethyl)-1-piperazinyl]-
15. Piribedil (inn)
16. 2-(4-(3,4-methylenedioxybenzyl)piperazino)pyrimidine
17. 1-(3,4-methylenedioxybenzyl)-4-(2-pyrimidyl)piperazine
18. Ncgc00015857-05
19. 2-(4-(1,3-benzodioxol-5-ylmethyl)-1-piperazinyl)pyrimidine
20. 2-[4-(1,3-benzodioxol-5-ylmethyl)-1-piperazinyl]pyrimidine
21. Piribedil [inn]
22. Pyrimidine, 2-(4-(1,3-benzodioxol-5-ylmethyl)-1-piperazinyl)-
23. Dsstox_cid_25188
24. Dsstox_rid_80735
25. Dsstox_gsid_45188
26. 2-[4-(2h-1,3-benzodioxol-5-ylmethyl)piperazin-1-yl]pyrimidine
27. Cas-3605-01-4
28. Piribedile [dcit]
29. Piribedil [inn:dcf]
30. Piribedilum [inn-latin]
31. Piribedile
32. Piribedilum
33. Sr-01000076091
34. Trivastal (tn)
35. Einecs 222-764-6
36. Brn 0963637
37. Tocris-1031
38. Piribedil [mi]
39. Prestwick0_000980
40. Prestwick1_000980
41. Prestwick2_000980
42. Prestwick3_000980
43. Unii-do22k1prdj
44. Lopac-p-9233
45. Biomol-nt_000044
46. Gtpl49
47. Piribedil [mart.]
48. Cambridge Id 5268291
49. Piribedil [who-dd]
50. Lopac0_000965
51. Oprea1_061309
52. Oprea1_215383
53. Bspbio_001019
54. Schembl150101
55. Spbio_002930
56. Bpbio1_001121
57. Bpbio1_001269
58. Chembl1371770
59. Dtxsid9045188
60. Bdbm85092
61. Chebi:92833
62. 2-(4-benzo[1,3]dioxol-5-ylmethyl-piperazin-1-yl)-pyrimidine
63. Hms2089m17
64. Hms3885e19
65. Bcp10553
66. Nsc_4850
67. Tox21_110245
68. Mfcd00868264
69. S3656
70. Stl497889
71. Zinc19537374
72. Akos001309525
73. Tox21_110245_1
74. Ac-1051
75. Ccg-205045
76. Cs-8014
77. Db12478
78. Es-0016
79. Sdccgsbi-0050938.p003
80. Ncgc00015857-01
81. Ncgc00015857-02
82. Ncgc00015857-03
83. Ncgc00015857-04
84. Ncgc00015857-06
85. Ncgc00015857-07
86. Ncgc00015857-09
87. Ncgc00015857-16
88. Ncgc00024951-01
89. Ncgc00024951-02
90. Ncgc00024951-03
91. Hy-12707
92. Cas_3605-01-4
93. Sbi-0050938.p002
94. Cas-78213-63-5
95. Ab00514645
96. Ft-0610865
97. P2054
98. 2,5-diamino-4-(dimethylamino)-4-nitrostilbene
99. D07305
100. T72361
101. Ab00514645-19
102. 605p014
103. A823101
104. L000476
105. Q413976
106. Brd-k47936004-001-01-9
107. Brd-k47936004-003-03-1
108. Sr-01000076091-11
109. Z31240640
110. 1-(2-pyrimidyl)-4-(3,4-methylenedioxybenzyl)piperazine
111. 2-{4-[(1,3-benzodioxol-5-yl)methyl]piperazin-1-yl}pyrimidine
112. 2-{4-[(2h-1,3-benzodioxol-5-yl)methyl]piperazin-1-yl}pyrimidine
113. 2-(4-(benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)pyrimidine Methanesulfonate
| Molecular Weight | 298.34 g/mol |
|---|---|
| Molecular Formula | C16H18N4O2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 298.14297583 g/mol |
| Monoisotopic Mass | 298.14297583 g/mol |
| Topological Polar Surface Area | 50.7 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 356 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
N04BC08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N04 - Anti-parkinson drugs
N04B - Dopaminergic agents
N04BC - Dopamine agonists
N04BC08 - Piribedil
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35392
Submission : 2020-11-24
Status : Active
Type : II
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 20980-22-7
End Use API : Piribedil
About The Company : PMC Isochem is a CDMO company acquired by PMC International in 2017. It manufactures cGMP intermediates, active pharmaceutical ingredients and functional excipi...
CAS Number : 20980-22-7
End Use API : Piribedil
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

CAS Number : 628-20-6
End Use API : Piribedil
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

CAS Number : 1075-89-4
End Use API : Piribedil
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

CAS Number : CAS-20980-22-7
End Use API : Piribedil
About The Company : Venkata Narayana Active Ingredients (Formerly Nutra Specialties Private Limited) Promoted by a well-known business house of India, Mr. Abhaya Kumar Jain who has...

CAS Number : CAS-1075-89-4
End Use API : Piribedil
About The Company : Venkata Narayana Active Ingredients (Formerly Nutra Specialties Private Limited) Promoted by a well-known business house of India, Mr. Abhaya Kumar Jain who has...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Spain
Brand Name :
Dosage Form : Extended Release Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Regulatory Info :
Registration Country : Italy
Brand Name : Trivastan
Dosage Form :
Dosage Strength : 30 Conf 20 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Trivastan
Dosage Form :
Dosage Strength : 30 Conf 50 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
47
PharmaCompass offers a list of Piribedil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Piribedil manufacturer or Piribedil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Piribedil manufacturer or Piribedil supplier.
PharmaCompass also assists you with knowing the Piribedil API Price utilized in the formulation of products. Piribedil API Price is not always fixed or binding as the Piribedil Price is obtained through a variety of data sources. The Piribedil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Piribedil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Piribedil, including repackagers and relabelers. The FDA regulates Piribedil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Piribedil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Piribedil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Piribedil supplier is an individual or a company that provides Piribedil active pharmaceutical ingredient (API) or Piribedil finished formulations upon request. The Piribedil suppliers may include Piribedil API manufacturers, exporters, distributors and traders.
click here to find a list of Piribedil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Piribedil DMF (Drug Master File) is a document detailing the whole manufacturing process of Piribedil active pharmaceutical ingredient (API) in detail. Different forms of Piribedil DMFs exist exist since differing nations have different regulations, such as Piribedil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Piribedil DMF submitted to regulatory agencies in the US is known as a USDMF. Piribedil USDMF includes data on Piribedil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Piribedil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Piribedil suppliers with USDMF on PharmaCompass.
A Piribedil written confirmation (Piribedil WC) is an official document issued by a regulatory agency to a Piribedil manufacturer, verifying that the manufacturing facility of a Piribedil active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Piribedil APIs or Piribedil finished pharmaceutical products to another nation, regulatory agencies frequently require a Piribedil WC (written confirmation) as part of the regulatory process.
click here to find a list of Piribedil suppliers with Written Confirmation (WC) on PharmaCompass.
Piribedil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Piribedil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Piribedil GMP manufacturer or Piribedil GMP API supplier for your needs.
A Piribedil CoA (Certificate of Analysis) is a formal document that attests to Piribedil's compliance with Piribedil specifications and serves as a tool for batch-level quality control.
Piribedil CoA mostly includes findings from lab analyses of a specific batch. For each Piribedil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Piribedil may be tested according to a variety of international standards, such as European Pharmacopoeia (Piribedil EP), Piribedil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Piribedil USP).
