

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDF
0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
Finished Drug Prices
NA
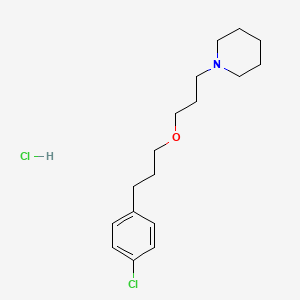

1. 903576-44-3
2. Ciproxidine
3. Bf 2649
4. Pitolisant Hcl
5. Pitolisant (hydrochloride)
6. Bf 2649 Hydrochloride
7. Yv33ch63hi
8. Pitolisant Hydrochloride [usan]
9. 1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine Hydrochloride
10. Piperidine, 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-, Hydrochloride
11. 903576-44-3 (hcl)
12. Wakix (tn)
13. Pitolisant Hydrochloride (usan)
14. 1-[3-[3-(4-chlorophenyl)propoxy]propyl]-piperidine Hydrochloride
15. 1-{3-[3-(4-chlorophenyl)propoxy]propyl}piperidine Hydrochloride
16. Piperidine, 1-(3-(3-(4-chlorophenyl)propoxy)propyl)-, Hydrochloride
17. Bf 2.649
18. Unii-yv33ch63hi
19. Schembl4591893
20. Bf-2649 Hydrochloride
21. Chembl4164059
22. Dtxsid50238098
23. Pitolisant;ciproxidine; Bf2649
24. Amy23423
25. Bcp16427
26. Ex-a1420
27. Hy-12199b
28. Pitolisant Hydrochloride [mi]
29. S5926
30. Akos024457784
31. Cs-1501
32. Sb17036
33. Pitolisant Hydrochloride [who-dd]
34. Ac-36017
35. As-72196
36. A3727
37. Pitolisant Hydrochloride [orange Book]
38. C77058
39. D11490
40. A900088
41. Q27895485
42. 1-[3-[3-(4-chlorophenyl)propoxy]propyl]piperidine;hydrochloride
43. 1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine Monohydrochloride
44. Piperidine, 1-(3-(3-(4-chlorophenyl)propoxy)propyl)-, Hydrochloride (1:1)
| Molecular Weight | 332.3 g/mol |
|---|---|
| Molecular Formula | C17H27Cl2NO |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 8 |
| Exact Mass | 331.1469699 g/mol |
| Monoisotopic Mass | 331.1469699 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 235 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-03-31
Pay. Date : 2023-03-15
DMF Number : 36859
Submission : 2022-03-01
Status : Active
Type : II

Date of Issue : 2024-02-20
Valid Till : 2026-12-06
Written Confirmation Number : WC-0416
Address of the Firm :

NDC Package Code : 58159-084
Start Marketing Date : 2021-06-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (35kg/35kg)
Marketing Category : BULK INGREDIENT
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-07-28
Pay. Date : 2023-07-06
DMF Number : 37753
Submission : 2022-12-31
Status : Active
Type : II

NDC Package Code : 73435-034
Start Marketing Date : 2023-12-20
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-05-19
Pay. Date : 2023-03-21
DMF Number : 38174
Submission : 2023-03-30
Status : Active
Type : II

NDC Package Code : 58159-084
Start Marketing Date : 2021-06-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (35kg/35kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-06-22
Pay. Date : 2023-04-21
DMF Number : 38307
Submission : 2023-05-09
Status : Active
Type : II

NDC Package Code : 70966-0038
Start Marketing Date : 2019-08-14
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-05-17
Pay. Date : 2023-04-25
DMF Number : 38131
Submission : 2023-03-27
Status : Active
Type : II

NDC Package Code : 14501-0114
Start Marketing Date : 2023-03-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2024-08-22
Pay. Date : 2024-07-25
DMF Number : 39126
Submission : 2023-12-05
Status : Active
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
GDUFA
DMF Review : Complete
Rev. Date : 2023-03-31
Pay. Date : 2023-03-15
DMF Number : 36859
Submission : 2022-03-01
Status : Active
Type : II
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
GDUFA
DMF Review : Complete
Rev. Date : 2023-05-19
Pay. Date : 2023-03-21
DMF Number : 38174
Submission : 2023-03-30
Status : Active
Type : II
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
GDUFA
DMF Review : Complete
Rev. Date : 2023-07-28
Pay. Date : 2023-07-06
DMF Number : 37753
Submission : 2022-12-31
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2023-06-22
Pay. Date : 2023-04-21
DMF Number : 38307
Submission : 2023-05-09
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2024-08-22
Pay. Date : 2024-07-25
DMF Number : 39126
Submission : 2023-12-05
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-05-17
Pay. Date : 2023-04-25
DMF Number : 38131
Submission : 2023-03-27
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Date of Issue : 2024-02-20
Valid Till : 2026-12-06
Written Confirmation Number : WC-0416
Address of the Firm : Plot No. 111 SIDCO Industrial Estate, Kakkalur Thiruvallur-602003 Tamil Nadu, In...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2029-09-26
US Patent Number : 8486947
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1102
Delist Requested :
Patent Use Description : METHOD OF TREATING CAT...
Patent Expiration Date : 2029-09-26

Patent Expiration Date : 2026-02-06
US Patent Number : 8354430
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1102
Delist Requested :
Patent Use Description : METHOD OF TREATING CAT...
Patent Expiration Date : 2026-02-06

Patent Expiration Date : 2026-02-06
US Patent Number : 8354430
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1102
Delist Requested :
Patent Use Description : METHOD OF TREATING CAT...
Patent Expiration Date : 2026-02-06

Patent Expiration Date : 2029-09-26
US Patent Number : 8486947
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1101
Delist Requested :
Patent Use Description : METHOD OF TREATING EXC...
Patent Expiration Date : 2029-09-26

Patent Expiration Date : 2029-09-26
US Patent Number : 8486947
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1102
Delist Requested :
Patent Use Description : METHOD OF TREATING CAT...
Patent Expiration Date : 2029-09-26

Patent Expiration Date : 2029-09-26
US Patent Number : 8486947
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1101
Delist Requested :
Patent Use Description : METHOD OF TREATING EXC...
Patent Expiration Date : 2029-09-26

Patent Expiration Date : 2026-02-06
US Patent Number : 8354430
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1101
Delist Requested :
Patent Use Description : METHOD OF TREATING EXC...
Patent Expiration Date : 2026-02-06

Patent Expiration Date : 2026-02-06
US Patent Number : 8354430
Drug Substance Claim :
Drug Product Claim :
Application Number : 211150
Patent Use Code : U-1101
Delist Requested :
Patent Use Description : METHOD OF TREATING EXC...
Patent Expiration Date : 2026-02-06

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2024-08-14
Application Number : 211150
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-255
Exclusivity Expiration Date : 2026-08-14
Application Number : 211150
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-331
Exclusivity Expiration Date : 2027-10-13
Application Number : 211150
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NPP
Exclusivity Expiration Date : 2027-06-21
Application Number : 211150
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-489
Exclusivity Expiration Date : 2031-06-21
Application Number : 211150
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NCE
Exclusivity Expiration Date : 2024-08-14
Application Number : 211150
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-255
Exclusivity Expiration Date : 2026-08-14
Application Number : 211150
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-331
Exclusivity Expiration Date : 2027-10-13
Application Number : 211150
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NPP
Exclusivity Expiration Date : 2027-06-21
Application Number : 211150
Product Number : 2
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : ODE-489
Exclusivity Expiration Date : 2031-06-21
Application Number : 211150
Product Number : 2
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
We have 5 companies offering Pitolisant Hydrochloride
Get in contact with the supplier of your choice:


LOOKING FOR A SUPPLIER?
