
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
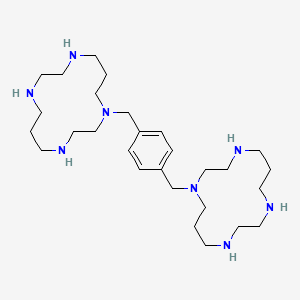

1. 1,1'-(1,4-phenylenebis(methylene))bis(1,4,8,11-tetraazacyclotetradecane)octahydrochloride Dihydrate
2. 1,1'-(1,4-phenylenebis-(methylene))-bis-1,4,8,11-tetraazacyclotetradecane
3. 1,1'-(1,4-phenylenebis-(methylene))-bis-1,4,8,11-tetraazacyclotetradecane Octahydrochloride Dihydrate
4. Amd 3100
5. Amd 3329
6. Amd-3100
7. Amd-3329
8. Amd3100
9. Jm 3100
10. Jm3100
11. Mezobil
12. Mozobil
13. Plerixafor Hydrochloride
14. Plerixafor Octahydrobromide
15. Plerixafor Octahydrochloride
16. Rpa Bicyclam
1. 110078-46-1
2. Mozobil
3. 1,4-bis((1,4,8,11-tetraazacyclotetradecan-1-yl)methyl)benzene
4. Amd3100
5. Amd 3100
6. Bicyclam Jm-2987
7. Plerixafor Hydrochloride
8. Sdz Sid 791
9. Plerixafor (amd3100)
10. Amd-3100
11. Jm3100
12. Jm 3100
13. 1-[[4-(1,4,8,11-tetrazacyclotetradec-1-ylmethyl)phenyl]methyl]-1,4,8,11-tetrazacyclotetradecane
14. Jkl 169
15. Jm-3100
16. Chembl18442
17. 1,1'-[1,4-phenylenebis(methylene)]bis-1,4,8,11-tetraazacyclotetradecane
18. S915p5499n
19. 1,1'-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane]
20. 1-{[4-(1,4,8,11-tetraazacyclotetradecan-1-ylmethyl)phenyl]methyl}-1,4,8,11-tetraazacyclotetradecane
21. 1,4,8,11-tetraazacyclotetradecane, 1,1'-(1,4-phenylenebis(methylene))bis-
22. Mozobil (tn)
23. Plerixafor [usan]
24. Plerixaforum
25. Plerixafor [usan:inn:ban]
26. Sid791
27. 1,1'-[1,4-phenylenebis(methylene)]bis(1,4,8,11-tetraazacyclotetradecane)
28. Unii-s915p5499n
29. 1,1'-(1,4-phenylenebis(methylene))bis-1,4,8,11-tetraazacyclotetradecane
30. Gna & Amd-3100
31. Hha & Amd-3100
32. Jm 2987
33. Plerixafor [mi]
34. Plerixafor [inn]
35. Plerixafor [jan]
36. Plerixafor [vandf]
37. Plerixafor [mart.]
38. Plerixafor-d4 (deuterated)
39. Plerixafor [who-dd]
40. Schembl19038
41. Gtpl844
42. Plerixafor (jan/usan/inn)
43. Plerixafor [ema Epar]
44. Plerixafor [orange Book]
45. Dtxsid70869520
46. Amd-3100 (cxcr4)
47. Chebi:125354
48. Bcpp000104
49. Bcp02337
50. Ex-a1762
51. Bdbm50035696
52. Mfcd05662218
53. Nsc754363
54. Nsc761388
55. S8030
56. Zinc22443609
57. 1,1'-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-tetraaza-cyclotetradecane}
58. Akos005266706
59. Ccg-269710
60. Cs-0451
61. Db06809
62. Nsc-754363
63. Nsc-761388
64. Ncgc00165722-01
65. Ncgc00165722-02
66. Ncgc00165722-03
67. 1,1'-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane] & Galanthus Nivalis Agglutinin (gna)
68. Ac-26837
69. As-42504
70. Hy-10046
71. Ft-0660392
72. Ft-0673966
73. P2678
74. A25446
75. D08971
76. Ab01566900_01
77. 078p461
78. A809618
79. L000104
80. Q905835
81. Sr-01000941593
82. J-503718
83. Sr-01000941593-1
84. 11''''-xylyl Bis-1,4,8,11-tetraazacyclotetradecane
85. Brd-k33240821-367-01-8
86. Z2196779619
87. 1,1''''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-tetraaza-cyclotetradecane}
88. 1,1'-(benzene-1,4-diyldimethanediyl)bis(1,4,8,11-tetraazacyclotetradecane)
89. 1,1'[1,4-phenylene-bis-(methylene)]-bis-1,4,8,11-tetraazacyclotetradecane
90. 1,1'-[1,4-phenylenebis(methylene)]bis[1,4,8,11-tetraazacyclotetradecane] & Hippeastrum Hybrid Agglutinin( Hha)
91. 1,4,8,11-tetraazacyclotetradecanyl[4-(1,4,8,11-tetraazacyclotetradecanylmethyl)phenyl]methane
92. 1,4,8,11-tetraazacyclotetradecanyl[4-(1,4,8,11-tetraazacyclotetradecanylmethyl)phenyl]methane(8hbr.2h2o)
93. 1,4-bis((1,4,8,11-tetraazacyclotetradecan-1-yl)methyl)benzene Octahydrochloride;plerixafor Octahydrochloride
94. 1-({4-[(1,4,8,11-tetraazacyclotetradecan-1-yl)methyl]phenyl}methyl)-1,4,8,11-tetraazacyclotetradecane
95. 11-{4-[4,8, 11-1,4,8,11tetraaza-cyclotetradec-1-ylmethyl]-benzyl}-1,4,8,11tetraaza-cyclotetradecane-1,4,8-tricarboxylic Acid Tri-tert-butyl Ester
| Molecular Weight | 502.8 g/mol |
|---|---|
| Molecular Formula | C28H54N8 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 502.44714376 g/mol |
| Monoisotopic Mass | 502.44714376 g/mol |
| Topological Polar Surface Area | 78.7 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 456 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Mozobil |
| PubMed Health | Plerixafor (Injection) |
| Drug Classes | Hematopoietic |
| Drug Label | Mozobil (plerixafor injection) is a sterile, preservative-free, clear, colorless to pale yellow, isotonic solution for subcutaneous injection. Each mL of the sterile solution contains 20 mg of plerixafor. Each single-use vial is filled to deliver 1... |
| Active Ingredient | Plerixafor |
| Dosage Form | Solution |
| Route | Subcutaneous |
| Strength | 24mg/1.2ml (20mg/ml) |
| Market Status | Prescription |
| Company | Genzyme |
| 2 of 2 | |
|---|---|
| Drug Name | Mozobil |
| PubMed Health | Plerixafor (Injection) |
| Drug Classes | Hematopoietic |
| Drug Label | Mozobil (plerixafor injection) is a sterile, preservative-free, clear, colorless to pale yellow, isotonic solution for subcutaneous injection. Each mL of the sterile solution contains 20 mg of plerixafor. Each single-use vial is filled to deliver 1... |
| Active Ingredient | Plerixafor |
| Dosage Form | Solution |
| Route | Subcutaneous |
| Strength | 24mg/1.2ml (20mg/ml) |
| Market Status | Prescription |
| Company | Genzyme |
Used in combination with granulocyte-colony stimulating factor (G-CSF, filgrastim) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkins lymphoma (NHL) and multiple myeloma (MM).
FDA Label
Mozobil is indicated in combination with granulocyte-colony-stimulating factor to enhance mobilisation of haematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with lymphoma and multiple myeloma whose cells mobilise poorly.
Plerixafor is a bicyclam derivative that antagonizes CXCR4 by binding to three acidic residues in the ligand-binding pocket: Asp171, Asp262, and Glu288. Blood levels of CD34+ cells peaked at 9 hours after administration of 0.24 mg/kg plerixafor in healthy subjects. In patients that have non-Hodgkins lymphoma or multiple myeloma, blood levels of CD34+ peaked at 6 hours. In combination with a G-CSF, circulating CD34+ cells in the peripheral blood peaked at 9-14 hours.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
L03AX16
L03AX16
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L03 - Immunostimulants
L03A - Immunostimulants
L03AX - Other immunostimulants
L03AX16 - Plerixafor
Absorption
Pharmacokinetic profile follows a two-compartment model with first-order absorption. A median peak plasma concentration of 0.24 mg/kg of plerixafor occurred 30-60 minutes after subcutaneous dose.
Route of Elimination
0.24 mg/kg, healthy subjects: ~70% of the parent drug is excreted in urine in the first 24 hours.
Volume of Distribution
0.3 L/kg
Clearance
Total plasma clearance: 4.38 L/h; Renal clearance: 3.15 L/h
Metabolism does not involved CYP isoenzymes
Terminal elimination half-life, NHL patients: 4.4 hours; Terminal elimination half-life, MM patients: 5.6 hours; Terminal elimination half-life, Hodgkin's lymphoma patients: 3.5 hours; Distribution half-life: 0.3 hours
Plerixafor inhibits the CXCR4 chemokine receptors on CD34+ cells and reversibly blocks binding of the ligand, stromal cell-derived factor-1-alpha (SDF-1). By blocking the interaction between SDF-1 and CXCR4 with plerixafor, mobilization of progenitor cells is triggered. Filgrastim, a granulocyte-colony stimulating factor, is added to enhance CD34+ cell mobilization, thus increasing the yield of stem cells- an important determinant of graft adequacy.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
16
PharmaCompass offers a list of Plerixafor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Plerixafor manufacturer or Plerixafor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Plerixafor manufacturer or Plerixafor supplier.
PharmaCompass also assists you with knowing the Plerixafor API Price utilized in the formulation of products. Plerixafor API Price is not always fixed or binding as the Plerixafor Price is obtained through a variety of data sources. The Plerixafor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Plerixafor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Plerixafor, including repackagers and relabelers. The FDA regulates Plerixafor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Plerixafor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Plerixafor manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Plerixafor supplier is an individual or a company that provides Plerixafor active pharmaceutical ingredient (API) or Plerixafor finished formulations upon request. The Plerixafor suppliers may include Plerixafor API manufacturers, exporters, distributors and traders.
click here to find a list of Plerixafor suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Plerixafor DMF (Drug Master File) is a document detailing the whole manufacturing process of Plerixafor active pharmaceutical ingredient (API) in detail. Different forms of Plerixafor DMFs exist exist since differing nations have different regulations, such as Plerixafor USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Plerixafor DMF submitted to regulatory agencies in the US is known as a USDMF. Plerixafor USDMF includes data on Plerixafor's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Plerixafor USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Plerixafor suppliers with USDMF on PharmaCompass.
A Plerixafor written confirmation (Plerixafor WC) is an official document issued by a regulatory agency to a Plerixafor manufacturer, verifying that the manufacturing facility of a Plerixafor active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Plerixafor APIs or Plerixafor finished pharmaceutical products to another nation, regulatory agencies frequently require a Plerixafor WC (written confirmation) as part of the regulatory process.
click here to find a list of Plerixafor suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Plerixafor as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Plerixafor API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Plerixafor as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Plerixafor and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Plerixafor NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Plerixafor suppliers with NDC on PharmaCompass.
Plerixafor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Plerixafor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Plerixafor GMP manufacturer or Plerixafor GMP API supplier for your needs.
A Plerixafor CoA (Certificate of Analysis) is a formal document that attests to Plerixafor's compliance with Plerixafor specifications and serves as a tool for batch-level quality control.
Plerixafor CoA mostly includes findings from lab analyses of a specific batch. For each Plerixafor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Plerixafor may be tested according to a variety of international standards, such as European Pharmacopoeia (Plerixafor EP), Plerixafor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Plerixafor USP).
