
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
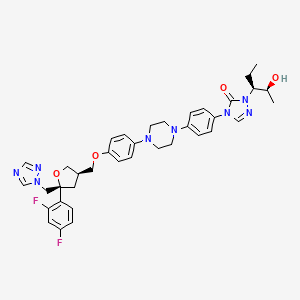

1. 4-(p-(4-(p-(((3r,5r)-5-(2,4-difluorophenyl)tetrahydro-5-(1h-1,2,4-triazol-1-ylmethyl)-3-furyl)methoxy)phenyl)-1-piperazinyl)phenyl)-1-((1s,2s)-1-ethyl-2-hydroxypropyl)-delta(sup 2)-1,2,4-triazolin-5-one
2. Noxafil
3. Posaconazole Hydrate
4. Sch 56592
5. Sch-56592
1. 171228-49-2
2. Noxafil
3. Sch 56592
4. Sch-56592
5. Posaconazole Sp
6. Sch56592
7. Schering 56592
8. 4-(4-(4-(4-(((3r,5r)-5-((1h-1,2,4-triazol-1-yl)methyl)-5-(2,4-difluorophenyl)tetrahydrofuran-3-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-1-((2s,3s)-2-hydroxypentan-3-yl)-1h-1,2,4-triazol-5(4h)-one
9. 4-[4-[4-[4-[[(3r,5r)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)oxolan-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(2s,3s)-2-hydroxypentan-3-yl]-1,2,4-triazol-3-one
10. 6tk1g07bhz
11. Chembl1397
12. Chebi:64355
13. 177571-33-4
14. 2,5-anhydro-1,3,4-trideoxy-2-(2,4-difluorophenyl)-4-({4-[4-(4-{1-[(2s,3s)-2-hydroxypentan-3-yl]-5-oxo-1,5-dihydro-4h-1,2,4-triazol-4-yl}phenyl)piperazin-1-yl]phenoxy}methyl)-1-(1h-1,2,4-triazol-1-yl)-d-threo-pentitol
15. D-threo-pentitol, 2,5-anhydro-1,3,4-trideoxy-2-c-(2,4-difluorophenyl)- 4-((4-(4-(4-(1-((1s,2s)-1-ety
16. Pos
17. Unii-6tk1g07bhz
18. Pasaconazole
19. Posaconazole [usan:inn:ban]
20. Hsdb 7421
21. 4-[4-[4-[4-[[(3r,5r)-5-(2,4-difluorophenyl)-5-(1,2,4-triazol-1-ylmethyl)tetrahydrofuran-3-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-2-[(1s,2s)-1-ethyl-2-hydroxy-propyl]-1,2,4-triazol-3-one
22. Noxafil (tn)
23. Posaconazole Solution
24. Posaconazole- Bio-x
25. Posaconazole - Form I
26. Posaconazole - Form Iii
27. Posaconazole [mi]
28. (-)-posaconazole
29. Posaconazole [inn]
30. Posaconazole [jan]
31. Posaconazole [hsdb]
32. Posaconazole [usan]
33. Posaconazole [vandf]
34. Posaconazole [mart.]
35. Posaconazole [who-dd]
36. Schembl991747
37. Posaconazole (jan/usan/inn)
38. Posaconazole [ema Epar]
39. Dtxsid6049066
40. Gtpl11428
41. Posaconazole [green Book]
42. Posaconazole [orange Book]
43. Amy30591
44. Bcp01102
45. Zinc3938482
46. Bdbm50181473
47. Mfcd00941162
48. Mmv688774
49. S1257
50. Akos005145917
51. Ac-1350
52. Ccg-270387
53. Cs-0998
54. Db01263
55. Ks-1413
56. Ncgc00274060-02
57. Ncgc00274060-07
58. 4-(p-(4-(p-(((3r,5r)-5-(2,4-difluorophenyl)tetrahydro-5-(1h-1,2,4-triazol-1-ylmethyl)-3-furyl)methoxy)phenyl)-1-piperazinyl)phenyl)-1-((1s,2s)-1-ethyl-2-hydroxypropyl)-delta(sup 2)-1,2,4-triazolin-5-one
59. Bp163592
60. Bp163593
61. Bp164281
62. Hy-17373
63. Posaconazole In Combination With Mgcd290
64. P2477
65. Sw219391-1
66. D02555
67. P-7020
68. Ab01274762-01
69. Ab01274762_02
70. Posaconazole, Vetranal(tm), Analytical Standard
71. 228p492
72. Q906453
73. Posaconazole, Antibiotic For Culture Media Use Only
74. Posaconazole Solution, 2.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
75. 1-((1s,2s)-1-ethyl-2-hydroxypropyl)-4-{4-[4-(4-{[(5s,3r)-5-(2,4-difluorophenyl)-5-(1,2,4-triazolylmethyl)oxolan-3-yl]methoxy}phenyl)piperazinyl]phenyl}-1,2,4-triazolin-5-one
76. 2,5-anhydro-1,3,4-trideoxy-2-(2,4-difluorophenyl)-4-({4-[4-(4-{1-[(1s,2s)-1-ethyl-2-hydroxypropyl]-5-oxo-1,5-dihydro-4h-1,2,4-triazol-4-yl}phenyl)piperazin-1-yl]phenoxy}methyl)-1-(1h-1,2,4-triazol-1-yl)-d-threo-pentitol
77. 3h-1,2,4-triazol-3-one, 4-(4-(4-(4-((5-(2,4-difluorophenyl)tetrahydro-5-(1h-1,2,4-triazol-1-ylmethyl)-3-furanyl)methoxy)phenyl)-1-piperazinyl)phenyl)-2-(1-ethyl-2-hydroxypropyl)-2,4-dihydro-, (3r-(3.alpha.(1s*,2s*),5.alpha.))-
78. 3h-1,2,4-triazol-3-one, 4-(4-(4-(4-((5-(2,4-difluorophenyl)tetrahydro-5-(1h-1,2,4-triazol-1-ylmethyl)-3-furanyl)methoxy)phenyl)-1-piperazinyl)phenyl)-2-(1-ethyl-2-hydroxypropyl)-2,4-dihydro-, (3r-(3alpha(1s*,2s*),5alpha))-
79. 4-(4-(4-(4-(((3r,5r)-5-((1h-1,2,4-triazol-1-yl)methyl)-5-(2,4-difluorophenyl)-tetrahydrofuran-3-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2-((2s,3s)-2-hydroxypentan-3-yl)-2h-1,2,4-triazol-3(4h)-one
80. 4-(4-(4-(4-(((3r,5r)-5-((1h-1,2,4-triazol-1-yl)methyl)-5-(2,4-difluorophenyl)tetrahydrofuran-3-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2-((2s,3s)-2-hydroxypentan-3-yl)-2,4-dihydro-3h-1,2,4-triazol-3-one
81. 4-(4-(4-(4-(((3r,5r)-5-(3,4-difluorophenyl)-5-(1h-1,2,4-triazol-1-yl)tetrahydrofuran-3-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-1-((2s,3s)-2-hydroxypentan-3-yl)-1h-1,2,4-triazol-5(4h)-one
82. 4-(4-(4-(4-(((5r)-5-((1h-1,2,4-triazol-1-yl)methyl)-5-(2,4-difluorophenyl)-tetrahydrofuran-3-yl)methoxy)phenyl)piperazin-1-yl)phenyl)-2-((2s,3s)-2-hydroxypentan-3-yl)-2h-1,2,4-triazol-3(4h)-one
83. 4-(p-(4-(p-(((3r,5r)-5-(2,4-difluorophenyl)tetrahydro-5-(1h-1,2,4-triazol-1-ylmethyl)-3-furyl)methoxy)phenyl)-1-piperazinyl)phenyl)-1-((1s,2s)-1-ethyl-2-hydroxypropyl)-.delta.(sup 2)-1,2,4-triazolin-5-one
84. 4-[4-(4-{4-[(r)-5-(2,4-difluoro-phenyl)-5-(4,5-dihydro-[1,2,4]triazol-1-ylmethyl)-tetrahydro-furan-3-ylmethoxy]-phenyl}-piperazin-1-yl)-phenyl]-2-((1s,2s)-1-ethyl-2-hydroxy-propyl)-2,4-dihydro-[1,2,4]triazol-3-one
85. D-threo-pentitol, 2,5-anhydro-1,3,4-trideoxy-2-c-(2,4-difluorophenyl)-4-((4-(4-(4-(1-((1s,2s)-1-etyl-2-hydroxypropyl)-1,5-dihydro-5-oxo-4h-1,2,4-triazol-4-yl)phenyl)-1-piperazinyl)phenoxy)methyl)-1-(1h-1,2,4-triazol-1-yl)-
86. D-threo-pentitol, 2,5-anhydro-1,3,4-trideoxy-2-c-(2,4-difluorophenyl)-4-[[4-[4-[4-[1-[(1s,2s)-1-ethyl-2-hydroxypropyl]-1,5-dihydro-5-oxo-4h-1,2,4-triazol-4-yl]phenyl]-1-piperazinyl]phenoxy]methyl]-1-( 1h-1,2,4-triazol-1-yl)-?
87. Pcz
| Molecular Weight | 700.8 g/mol |
|---|---|
| Molecular Formula | C37H42F2N8O4 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 12 |
| Exact Mass | 700.32970817 g/mol |
| Monoisotopic Mass | 700.32970817 g/mol |
| Topological Polar Surface Area | 112 Ų |
| Heavy Atom Count | 51 |
| Formal Charge | 0 |
| Complexity | 1170 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Noxafil |
| PubMed Health | Posaconazole |
| Drug Classes | Antifungal |
| Drug Label | Noxafil is an azole antifungal agent available as concentrated solution to be diluted before intravenous administration, delayed-release tablet, or suspension for oral administration.Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (... |
| Active Ingredient | Posaconazole |
| Dosage Form | Suspension; Tablet, delayed release; Solution |
| Route | oral; Iv (infusion); Oral |
| Strength | 300mg/16.7ml (18mg/ml); 100mg; 40mg/ml |
| Market Status | Prescription |
| Company | Merck Sharp Dohme; Schering |
| 2 of 2 | |
|---|---|
| Drug Name | Noxafil |
| PubMed Health | Posaconazole |
| Drug Classes | Antifungal |
| Drug Label | Noxafil is an azole antifungal agent available as concentrated solution to be diluted before intravenous administration, delayed-release tablet, or suspension for oral administration.Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (... |
| Active Ingredient | Posaconazole |
| Dosage Form | Suspension; Tablet, delayed release; Solution |
| Route | oral; Iv (infusion); Oral |
| Strength | 300mg/16.7ml (18mg/ml); 100mg; 40mg/ml |
| Market Status | Prescription |
| Company | Merck Sharp Dohme; Schering |
Mesh Heading: Antibiotics, antifungals, trypanocidal agents
National Library of Medicine, SIS; ChemIDplus Record for Posaconazole (171228-49-2). Available from, as of April 14, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION: Antifungal; Orally activated triazole antifungal
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1365
The pharmacokinetics of posaconazole oral suspension in neutropenic patients undergoing high-dose chemotherapy and stem cell transplantation were evaluated, and the association of plasma posaconazole exposure with the presence and severity of oral mucositis was explored in this nonrandomized, open-label, parallel-group, multiple-dose pharmacokinetic study. Thirty patients were enrolled and received one of three regimens (group I, 200 mg once daily; group II, 400 mg once daily; group III, 200 mg four times daily) for the duration of neutropenia. The mean total exposure for day 1, as shown by the area under the concentration-time curve from 0 to 24 h (AUC(0-24)), was 1.96 mg . h/liter in group I and was 51% higher in group II and in group III. Increases in AUC(0-24) and maximum plasma concentration (C(max)) in groups II and III were dose related. The AUC(0-24) and C(max) values on day 1 were similar between groups II and III. There was interpatient variability of up to 68% in the pharmacokinetic values for our study population. Steady state was attained by days 5 to 6. Average steady-state plasma posaconazole trough values were 192, 219, and 414 ng/ml in groups I, II, and III, respectively. The AUC(0-24) and apparent oral clearance increased by increasing dose and dosing frequency. Mucositis appeared to reduce exposure but did not significantly affect mean total posaconazole exposure (AUC and C(max)) at steady state (P = 0.1483). Moreover, this reduction could be overcome by increasing the total dose and dosing frequency. Posaconazole was safe and well tolerated.
PMID:16723557 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1479147 Gubbins PO et al; Antimicrob Agents Chemother 50 (6): 1993-9 (2006)
/EXPTL:/ ... Posaconazole has demonstrated strong antifungal efficacy in Phase II and III clinical trials in immunocompromised patients with oropharyngeal and esophageal candidiasis. Posaconazole also showed promising efficacy as salvage therapy in a large Phase II study including 330 patients with invasive fungal infections intolerant to or refractory to standard therapies. ...
PMID:16107193 Groll AH et al; Expert Rev Anti Infect Ther 3 (4): 467-87 (2005)
Invasive fungal infections are found most frequently in immunosuppressed and critically ill hospitalized patients. Antifungal therapy is often required for long periods. Safety data from the clinical development program of the triazole antifungal agent, posaconazole, were analyzed. A total of 428 patients with refractory invasive fungal infections (n = 362) or febrile neutropenia (n = 66) received posaconazole in 2 phase II/III open-label clinical trials. Also, 109 of these patients received posaconazole therapy for > or = 6 months. Incidences of treatment-emergent, treatment-related, and serious adverse events and abnormal laboratory parameters were recorded during these studies. Treatment-emergent, treatment-related adverse events were reported in 38% of the overall patient population. The most common treatment-related adverse events were nausea (8%) and vomiting (6%). Treatment-related serious adverse events occurred in 8% of patients. Low rates of treatment-related corrected QT interval and/or QT interval prolongation (1%) and elevation of hepatic enzymes (2%) were reported as adverse events. Treatment-emergent, treatment-related adverse events occurred at similar rates in patients who received posaconazole therapy for < 6 months and > or = 6 months. Prolonged posaconazole treatment was associated with a generally favorable safety profile in seriously ill patients with refractory invasive fungal infections. Long-term therapy did not increase the risk of any individual adverse event, and no unique adverse event was observed with longer exposure to posaconazole.
PMID:16705579 Raad II et al; Clin Infect Dis 42 (12): 1726-34 (2006)
The pharmacokinetic profiles, safety, and efficacies of different dosing schedules of posaconazole oral suspension in patients with possible, probable, and proven refractory invasive fungal infection (rIFI) or febrile neutropenia (FN) were evaluated in a multicenter, open-label, parallel-group study. Sixty-six patients with FN and 32 patients with rIFI were randomly assigned to one of three posaconazole regimens: 200 mg four times a day (q.i.d.) for nine doses, followed by 400 mg twice a day (b.i.d.); 400 mg q.i.d. for nine doses, followed by 600 mg b.i.d.; or 800 mg b.i.d. for five doses, followed by 800 mg once a day (q.d.). Therapy was continued for up to 6 months in patients with rIFI or until neutrophil recovery occurred in patients with FN. The 400-mg-b.i.d. dose provided the highest overall mean exposure, with 135% (P = 0.0004) and 182% (P < 0.0001) greater exposure than the 600-mg-b.i.d. and 800-mg-q.d. doses, respectively. However, exposure in allogeneic bone marrow transplant (BMT) recipients (n = 12) was 52% lower than in non-BMT patients. Treatment-related adverse events (occurring in 24% of patients) were mostly gastrointestinal in nature. Twenty-four percent of patients had adverse events leading to premature discontinuation (none were treatment related). In efficacy-evaluable patients, successful clinical response was observed in 43% with rIFI (56% of patients receiving 400 mg b.i.d., 17% receiving 600 mg b.i.d., and 50% receiving 800 mg q.d.) and 77% with FN (74% receiving 400 mg b.i.d., 78% receiving 600 mg b.i.d., and 81% receiving 800 mg q.d.). Posaconazole is well tolerated and absorbed. Divided doses of 800 mg (400 mg b.i.d.) provide the greatest posaconazole exposure.
PMID:16436724 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1366875 Ullmann AJ et al; Antimicrob Agents Chemother 50 (2): 658-66 (2006)
The authors evaluated the pharmacokinetics and safety of posaconazole in healthy subjects and in those with mild (CL(CR) = 50-80 mL/min), moderate (CL(CR) = 20-49 mL/min), and severe chronic renal disease (CL(CR) <20 mL/min; receiving outpatient hemodialysis) (n = 6/group). Subjects received one 400-mg dose of posaconazole oral suspension with a standardized high-fat breakfast. For hemodialysis-dependent subjects, this dose was given on a nonhemodialysis day, and a second 400-mg dose was given 6 hours before hemodialysis. ...There was no correlation between posaconazole pharmacokinetics and mild to moderate renal disease ...Furthermore, the difference in the predialyzed and postdialyzed posaconazole concentrations was only approximately 3%, supporting that posaconazole was not removed by hemodialysis. ...
PMID:15647411 Courtney R et al; J Clin Pharmacol 45 (2): 185-92 (2005)
For prophylaxis of invasive Aspergillus and Candida infections in patients, 13 years of age and older, who are at high risk of developing these infections due to being severely immunocompromised as a result of procedures such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD), or due to hematologic malignancies with prolonged neutropenia from chemotherapy. Also for the treatment of oropharyngeal candidiasis, including oropharyngeal candidiasis refractory to itraconazole and/or fluconazole. Posaconazole is used as an alternative treatment for invasive aspergillosis, Fusarium infections, and zygomycosis in patients who are intolerant of, or whose disease is refractory to, other antifungals.
FDA Label
Noxafil gastro-resistant tablets are indicated for use in the treatment of the following fungal infections in adults (see sections 4. 2 and 5. 1):
- Invasive aspergillosis
Noxafil gastro-resistant tablets are indicated for use in the treatment of the following fungal infections in paediatric patients from 2 years of age weighing more than 40 kg and adults (see sections 4. 2 and 5. 1):
- Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Noxafil gastro-resistant tablets are also indicated for prophylaxis of invasive fungal infections in the following paediatric patients from 2 years of age weighing more than 40 kg and adults (see sections 4. 2 and 5. 1):
- Patients receiving remission-induction chemotherapy for acute myelogenous leukaemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at high risk of developing invasive fungal infections;
- Hematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease and who are at high risk of developing invasive fungal infections.
Please refer to the Summary of Product Characteristics of Noxafil oral suspension for use in oropharyngeal candidiasis.
Noxafil concentrate for solution for infusion is indicated for use in the treatment of the following fungal infections in adults (see sections 4. 2 and 5. 1):
- Invasive aspergillosis
Noxafil concentrate for solution for infusion is indicated for use in the treatment of the following fungal infections in adult and paediatric patients from 2 years of age (see sections 4. 2 and 5. 1):
- Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Noxafil concentrate for solution for infusion is also indicated for prophylaxis of invasive fungal infections in the following adult and paediatric patients from 2 years of age (see sections 4. 2 and 5. 1):
- Patients receiving remission-induction chemotherapy for acute myelogenous leukaemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at high risk of developing invasive fungal infections;
- Hematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease (GVHD) and who are at high risk of developing invasive fungal infections.
Please refer to the Summary of Product Characteristics of Noxafil oral suspension for use in oropharyngeal candidiasis.
Noxafil gastro resistant powder and solvent for oral suspension is indicated for use in the treatment of the following fungal infections in paediatric patients from 2 years of age (see sections 4. 2 and 5. 1):
- Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Noxafil gastro-resistant powder and solvent for oral suspension is indicated for prophylaxis of invasive fungal infections in the following paediatric patients from 2 years of age:
- Patients receiving remission-induction chemotherapy for acute myelogenous leukaemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at high risk of developing invasive fungal infections;
- Haematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease and who are at high risk of developing invasive fungal infections.
Please refer to the Summary of Product Characteristics of Noxafil concentrate for solution for infusion and the gastro-resistant tablets for use in primary treatment of invasive aspergillosis.
Please refer to the Summary of Product Characteristics of Noxafil oral suspension for use in oropharyngeal candidiasis.
Noxafil oral suspension is indicated for use in the treatment of the following fungal infections in adults (see section 5. 1):
- Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products;
- Oropharyngeal candidiasis: as first-line therapy in patients who have severe disease or are immunocompromised, in whom response to topical therapy is expected to be poor.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Noxafil oral suspension is also indicated for prophylaxis of invasive fungal infections in the following patients:
- Patients receiving remission-induction chemotherapy for acute myelogenous leukaemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at high risk of developing invasive fungal infections;
- Hematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease and who are at high risk of developing invasive fungal infections.
Please refer to the Summary of Product Characteristics of Noxafil concentrate for solution for infusion and the gastro-resistant tablets for use in primary treatment of invasive aspergillosis.
Posaconazole Accord is indicated for use in the treatment of the following fungal infections in adults:
- Invasive aspergillosis;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Posaconazole Accord is also indicated for prophylaxis of invasive fungal infections in the following patients:
- Patients receiving remission-induction chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at high risk of developing invasive fungal infections;
- Hematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease and who are at high risk of developing invasive fungal infections.
Posaconazole AHCL oral suspension is indicated for use in the treatment of the following fungal infections in adults:
- Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products.
- Oropharyngeal candidiasis: as first-line therapy in patients who have severe disease or are immunocompromised, in whom response to topical therapy is expected to be poor.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Posaconazole AHCL oral suspension is also indicated for prophylaxis of invasive fungal infections in the following patients:
- Patients receiving remission-induction chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are at high risk of developing invasive fungal infections;
- Hematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease and who are at high risk of developing invasive fungal infections.
Posaconazole SP is indicated for use in the treatment of the following fungal infections in adults (see section 5. 1):
- Invasive aspergillosis in patients with disease that is refractory to amphotericin B or itraconazole or in patients who are intolerant of these medicinal products;
- Fusariosis in patients with disease that is refractory to amphotericin B or in patients who are intolerant of amphotericin B;
- Chromoblastomycosis and mycetoma in patients with disease that is refractory to itraconazole or in patients who are intolerant of itraconazole;
- Coccidioidomycosis in patients with disease that is refractory to amphotericin B, itraconazole or fluconazole or in patients who are intolerant of these medicinal products;
- Oropharyngeal candidiasis: as first-line therapy in patients who have severe disease or are immunocompromised, in whom response to topical therapy is expected to be poor.
Refractoriness is defined as progression of infection or failure to improve after a minimum of 7 days of prior therapeutic doses of effective antifungal therapy.
Posaconazole SP is also indicated for prophylaxis of invasive fungal infections in the following patients:
- Patients receiving remission-induction chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) expected to result in prolonged neutropenia and who are
at high risk of developing invasive fungal infections;
- Hematopoietic stem cell transplant (HSCT) recipients who are undergoing high-dose immunosuppressive therapy for graft versus host disease and who are at high risk of developing invasive fungal infections.
Prevention of invasive fungal infections, Treatment of invasive fungal infections
Posaconazole is an antifungal agent structurally related to itraconazole. It is a drug derived from itraconzaole through the replacement of the chlorine substituents with flourine in the phenyl ring, as well as hydroxylation of the triazolone side chain. These modifications enhance the potency and spectrum of activity of the drug. Posaconazole can be either fungicial or fungistatic in action.
Trypanocidal Agents
Agents destructive to the protozoal organisms belonging to the suborder TRYPANOSOMATINA. (See all compounds classified as Trypanocidal Agents.)
14-alpha Demethylase Inhibitors
Compounds that specifically inhibit STEROL 14-DEMETHYLASE. A variety of azole-derived ANTIFUNGAL AGENTS act through this mechanism. (See all compounds classified as 14-alpha Demethylase Inhibitors.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
J02AC04
J02AC04
J02AC04
J02AC04
J - Antiinfectives for systemic use
J02 - Antimycotics for systemic use
J02A - Antimycotics for systemic use
J02AC - Triazole and tetrazole derivatives
J02AC04 - Posaconazole
Absorption
Posaconazole is absorbed with a median Tmax of approximately 3 to 5 hours.
Route of Elimination
The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Volume of Distribution
1774 L
Clearance
32 L/hr
51 L/hr [Single-Dose Suspension Administration of 200 mg, fasted]
21 L/hr [Single-Dose Suspension Administration of 200 mg, nonfat meal]
14 L/hr [Single-Dose Suspension Administration of 200 mg, high fat meal]
91 L/hr [Single-Dose Suspension Administration of 400 mg, fasted]
43 L/hr [Single-Dose Suspension Administration of 400 mg with liquid nutritional supplement (14 g fat)]
Kinetics and protein binding following oral posaconazole dosing were performed in neutropenic infected mice. Peak levels and AUC from 0 hr to infinity values were nonlinear over the 16-fold dose range studied. Serum drug elimination half-life ranged from 12.0 to 17.7 hr
PMID:14693531 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC310197 Andes D et al; Antimicrob Agents Chemother 48 (1): 137-42 (2004)
The suspension formulation of posaconazole was associated with enhanced systemic exposure and increased relative bioavailability compared with the tablet. Food substantially enhanced the rate and extent of posaconazole absorption in healthy subjects.
Courtney R et al; Br J Clin Pharmacol. 2004 Feb;57(2):218-22
A total of 103 healthy adults were enrolled in two phase I trials. Each study had a double-blind, placebo-controlled, parallel-group design with a rising single-dose (RSD) or rising multiple-dose (RMD) scheme. In the RSD study, subjects received single doses of posaconazole oral tablets (50 to 1200 mg) or placebo. In the RMD study, subjects received posaconazole oral tablets (50 to 400 mg) or placebo twice daily for 14 days. By using model-independent methods, the area under the plasma concentration-time curve and the maximum concentration in plasma were determined and used to assess dose proportionality. In the RSD study, the levels of posaconazole in plasma increased proportionally between the 50- and 800-mg dose range, with saturation of absorption occurring above 800 mg. Dose proportionality was also observed in the RMD study. In both studies, the apparent volume of distribution was large (range, 343 to 1341 liters) and the terminal-phase half-life was long (range, 25 to 31 hr).
PMID:12936975 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC182636 Courtney R et al; Antimicrob Agents Chemother 47 (9): 2788-95 (2003)
Subjects fasted 12 hours before and 48 hours after the administration of posaconazole oral suspension (800 mg) given as a single dose (regimen A), 400 mg every 12 hours (regimen B) or 200 mg every 6 hours (regimen C). Plasma posaconazole concentrations were determined for 48 hours after the initial dose and subjects completed a 1-week washout period between treatment regimens. A one-compartment oral model with first-order rate of absorption and first-order rate of elimination was fitted to the plasma concentration-time data. Differences in exposure were investigated by allowing the bioavailability fraction to vary among regimens. A total of 18 healthy men were enrolled in and completed the study. : Posaconazole relative bioavailability was estimated to be significantly different among regimens (p < 0.0001) and increased with the number of doses, such that regimen B/regimen A = 1.98 +/- 0.35, representing a 98% increase, and regimen C/regimen A = 3.20 +/- 0.69, or a 220% increase. With use of the one-compartment model, the population steady-state values for area under the concentration-time curve over 24 hours were predicted to be 3900, 7700 and 12 400 microg.h/L, with average plasma concentrations of 162, 320 and 517 microg/L for regimens A, B and C, respectively. These data suggest that divided daily dose administration (every 12 or 6 hours) significantly increases posaconazole exposure under fasted conditions.
PMID:15656699 Ezzet F et al; Clin Pharmacokinet 44 (2): 211-20 (2005)
For more Absorption, Distribution and Excretion (Complete) data for POSACONAZOLE (6 total), please visit the HSDB record page.
Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Posaconazole is eliminated with a mean half-life (t½) of 35 hours (range 20 to 66 hours).
The i.v. terminal-phase half-lives were 7 hr in mice and rats, 15 hr in dogs, and 23 hr in monkeys. In rabbits, the oral half-life was 9 hr.
PMID:10681346 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC89754 Nomeir AA et al; Antimicrob Agents Chemother 44 (3): 727-31 (2000)
Kinetics and protein binding following oral posaconazole dosing were performed in neutropenic infected mice. Peak levels and AUC from 0 hr to infinity values were nonlinear over the 16-fold dose range studied. Serum drug elimination half-life ranged from 12.0 to 17.7 hr
PMID:14693531 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC310197 Andes D et al; Antimicrob Agents Chemother 48 (1): 137-42 (2004)
As a triazole antifungal agent, posaconazole exerts its antifungal activity through blockage of the cytochrome P-450 dependent enzyme, sterol 14α-demethylase, in fungi by binding to the heme cofactor located on the enzyme. This leads to the inhibition of the synthesis of ergosterol, a key component of the fungal cell membrane, and accumulation of methylated sterol precursors. This results in inhibition of fungal cell growth and ultimately, cell death.
Posaconazole is a novel lipophilic antifungal triazole that inhibits cytochrome P450-dependent 14-alpha demethylase in the biosynthetic pathway of ergosterol. Inhibition of this enzyme leads to an accumulation of toxic 14-alpha methylsterols and a depletion of ergosterol, resulting in a perturbation of the function of the fungal cell membrane and blockage of cell growth and division. ...
PMID:16107193 Groll AH et al; Expert Rev Anti Infect Ther 3 (4): 467-87 (2005)
... The primary mechanism of action of the drug was inhibition of the parasite's sterol C-14 alpha demethylase.
PMID:9661019 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC105681 Urbina JA et al; Antimicrob Agents Chemother 42 (7): 1771-7 (1998)
The in vitro activity of the novel triazole antifungal agent posaconazole was assessed in 45 laboratories against approximately 19,000 clinically important strains of yeasts and molds. The activity of posaconazole was compared with those of itraconazole, fluconazole, voriconazole, and amphotericin B against subsets of the isolates. Strains were tested utilizing Clinical and Laboratory Standards Institute broth microdilution methods using RPMI 1640 medium (except for amphotericin B, which was frequently tested in antibiotic medium 3). MICs were determined at the recommended endpoints and time intervals. Against all fungi in the database (22,850 MICs), the MIC(50) and MIC(90) values for posaconazole were 0.063 microg/ml and 1 mug/ml, respectively. MIC(90) values against all yeasts (18,351 MICs) and molds (4,499 MICs) were both 1 mug/ml. In comparative studies against subsets of the isolates, posaconazole was more active than, or within 1 dilution of, the comparator drugs itraconazole, fluconazole, voriconazole, and amphotericin B against approximately 7,000 isolates of Candida and Cryptococcus spp. Against all molds (1,702 MICs, including 1,423 MICs for Aspergillus isolates), posaconazole was more active than or equal to the comparator drugs in almost every category. Posaconazole was active against isolates of Candida and Aspergillus spp. that exhibit resistance to fluconazole, voriconazole, and amphotericin B and was much more active than the other triazoles against zygomycetes. Posaconazole exhibited potent antifungal activity against a wide variety of clinically important fungal pathogens and was frequently more active than other azoles and amphotericin B.
PMID:16723559 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1479149 Sabatelli F et al; Antimicrob Agents Chemother 50 (6): 2009-15 (2006)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?