
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA

1. Boron Gluconate
2. D-gluconate
3. D-gluconic Acid
4. Dextronic Acid
5. Gluconate
6. Gluconic Acid
7. Gluconic Acid, (113)indium-labeled
8. Gluconic Acid, (14)c-labeled
9. Gluconic Acid, (159)dysprosium-labeled Salt
10. Gluconic Acid, (99)technecium (5+) Salt
11. Gluconic Acid, 1-(14)c-labeled
12. Gluconic Acid, 6-(14)c-labeled
13. Gluconic Acid, Aluminum (3:1) Salt
14. Gluconic Acid, Ammonium Salt
15. Gluconic Acid, Calcium Salt
16. Gluconic Acid, Cesium(+3) Salt
17. Gluconic Acid, Cobalt (2:1) Salt
18. Gluconic Acid, Copper Salt
19. Gluconic Acid, Fe(+2) Salt, Dihydrate
20. Gluconic Acid, Lanthanum(+3) Salt
21. Gluconic Acid, Magnesium (2:1) Salt
22. Gluconic Acid, Manganese (2:1) Salt
23. Gluconic Acid, Monolithium Salt
24. Gluconic Acid, Monopotassium Salt
25. Gluconic Acid, Monosodium Salt
26. Gluconic Acid, Potassium Salt
27. Gluconic Acid, Sodium Salt
28. Gluconic Acid, Strontium (2:1) Salt
29. Gluconic Acid, Tin(+2) Salt
30. Gluconic Acid, Zinc Salt
31. Lithium Gluconate
32. Magnerot
33. Magnesium Gluconate
34. Maltonic Acid
35. Manganese Gluconate
36. Pentahydroxycaproic Acid
37. Sodium Gluconate
38. Zinc Gluconate
1. 299-27-4
2. Potassium D-gluconate
3. Gluconic Acid Potassium Salt
4. D-gluconic Acid, Monopotassium Salt
5. Monopotassium D-gluconate
6. Potassium (2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoate
7. D-gluconic Acid Potassium Salt
8. Kali Gluconicum
9. Hsdb 3165
10. D-gluconic Acid, Potassium Salt
11. 12h3k5qkn9
12. Ins No.577
13. Potassuril
14. Ins-577
15. Kaon Elixir
16. Potassiumd-gluconate
17. Katorin
18. Potalium
19. Potasoral
20. Sirokal
21. Kalium-beta
22. E-577
23. Gluconsan K
24. Kalium Gluconate
25. Potassiumgluconate
26. K-iao
27. Gluconic Acid, Monopotassium Salt
28. Einecs 206-074-2
29. Unii-12h3k5qkn9
30. Potassium Gluconate [usp:jan]
31. D-gluconic Acid, Potassium Salt (1:1)
32. Einecs 252-355-8
33. Gluconsan-k (tn)
34. Mfcd00064211
35. Gluconic Acid, Monopotassium Salt, D-
36. D-gluconic Acid, Potassium Salt (1:?)
37. Dsstox_cid_9617
38. Ec 206-074-2
39. Dsstox_rid_78789
40. Gluconic Acid (food Grade)
41. Dsstox_gsid_29617
42. Schembl40567
43. 2,3,4,5,6-pentahydroxycaproic Acid Potassium Salt
44. Potassium Gluconate (jan/usp)
45. Chembl2106978
46. Dtxsid7029617
47. Potassium D-gluconate, >=99%
48. Chebi:32032
49. Gluconic Acid (technical Grade)
50. Hy-y0569c
51. Potassium Gluconate [fcc]
52. Potassium Gluconate [jan]
53. Potassium Gluconate [hsdb]
54. Potassium Gluconate [inci]
55. Potassium Gluconate [vandf]
56. Potassium Gluconate [mart.]
57. Tox21_202774
58. Potassium Gluconate [usp-rs]
59. Potassium Gluconate [who-dd]
60. Akos000277995
61. Db13620
62. Ncgc00260321-01
63. Ac-15968
64. As-83530
65. Cas-299-27-4
66. Gluconic Acid Potassium Salt [mi]
67. Potassium Gluconate [usp Monograph]
68. Cs-0108842
69. G0040
70. D01298
71. A876352
72. Q1122870
73. W-202242
74. Potassium Gluconate, Meets Usp Testing Specifications, Anhydrous
75. Potassium;(2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoate
76. D-gluconic Acid Monopotassium Salt; Gluconic Acid Potassium Salt;
77. Potassium Gluconate, United States Pharmacopeia (usp) Reference Standard
78. Potassium Gluconate, Pharmaceutical Secondary Standard; Certified Reference Material
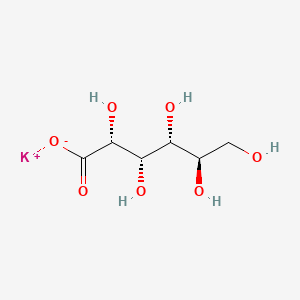
| Molecular Weight | 234.25 g/mol |
|---|---|
| Molecular Formula | C6H11KO7 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 234.01418417 g/mol |
| Monoisotopic Mass | 234.01418417 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 176 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
IRRESPECTIVE OF THE SALT USED, POTASSIUM IS COMPLETELY DISSOCIABLE & HENCE IS UNAFFECTED IN ITS IRRITANT ACTIONS & ABSORPTION BY THE ANION IN THE COMPD. /POTASSIUM SALTS/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 771
A SOURCE OF POTASSIUM FOR MGMNT OF HYPOKALEMIC STATES, SUCH AS OCCUR CONSEQUENT TO ADRENOCORTICOID THERAPY OR USE OF THIAZIDE DIURETICS, OR FOR DELIBERATE PRODN OF HYPERKALEMIA, AS FOR TREATMENT OF DIGITALIS INTOXICATION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 771
...USED TO TREAT HYPOKALEMIA ASSOC WITH HYPERCHLOREMIA (EG RENAL TUBULAR ACIDOSIS, HYPOKALEMIA ASSOC WITH ACIDOSIS). IF...USED IN PT WITH HYPOKALEMIC HYPOCHLOREMIC ALKALOSIS, A SOURCE OF CHLORIDE ION (EG, AMMONIUM CHLORIDE, LYSINE MONOHYDROCHLORIDE) SHOULD BE PROVIDED. /POTASSIUM PREPN/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 238
SUGAR-COATED POTASSIUM GLUCONATE TABLETS DISSOLVE @ HIGHER LEVEL /IN GI TRACT/ THAN DO ENTERIC-COATED TABLETS OF POTASSIUM CHLORIDE BUT, BY THIS VERY FACT, ARE FREE TO CAUSE THE IRRITATION FOR WHICH CHLORIDE TABLET WAS COATED. /THUS/...GLUCONATE HAS NO ADVANTAGE OVER NONENTERIC-COATED POTASSIUM CHLORIDE TABLETS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 771
A FULL GLASS OF WATER TAKEN WITH /POTASSIUM GLUCONATE/...GREATLY REDUCES THE IRRITANT EFFECTS... HYPOCHLOREMIA IS FREQUENT ACCOMPANIMENT OF HYPOKALEMIA; IN SUCH INSTANCES /POTASSIUM/ CHLORIDE IS DEFINITELY PREFERRED /OVER POTASSIUM GLUCONATE/.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 771
...SINCE GLUCONATE METABOLIZES TO BICARBONATE, IT CONTRIBUTES TO ALKALOSIS, WHICH MAY BE...PRESENT IN HYPOKALEMIA. THUS IT WOULD BE DIFFICULT TO FIND SITUATIONS IN WHICH GLUCONATE WOULD BE SUPERIOR /TO POTASSIUM CHLORIDE/.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 771
Because of potassiums wide-ranging roles in the body, low intakes can increase the risk of illness. Potassium supplements are indicated to prevent hypokalemia in patients who would be at particular risk if hypokalemia were to develop (e.g., digitalis treated patients with significant cardiac arrhythmias). Potassium deficiency occurs when the rate of loss through renal excretion and/or loss from the gastrointestinal tract is higher than the rate of potassium intake. In addition to serving as a preventative supplement, potassium gluconate also serves as a treatment for decreased potassium levels,,.
Potassium is an essential nutrient. It is the most abundant cation in intracellular fluid, where it plays a key role in maintaining cell function, especially in excitable cells such as skeletal muscles, the heart, and nerves. Increases in interstitial potassium play an important role in eliciting rapid vasodilation, allowing for blood flow to increase in exercising muscle.
A - Alimentary tract and metabolism
A12 - Mineral supplements
A12B - Potassium
A12BA - Potassium
A12BA05 - Potassium gluconate
Absorption
Potassium is rapidly and well absorbed. A 2016 dose-response trial found that humans absorb about 94% of potassium gluconate in supplements, and this absorption rate is similar to that of potassium from potatoes.
Route of Elimination
90% of potassium is eliminated via the kidneys. A small amount is eliminated in feces and sweat.
Volume of Distribution
Distribution is largely intracellular, but it is the intravascular concentration that is primarily responsible for toxicity.
Clearance
Potassium is freely filtered by the glomerulus in the kidney. The majority of filtered potassium is reabsorbed in the proximal tubule and loop of Henle. Less than 10% of the filtered load reaches the distal nephron. In the proximal tubule of the nephron, potassium absorption is mainly passive and proportional to Na+ and water. K+ reabsorption in the thick ascending limb of Henle occurs through both transcellular and paracellular pathways. The transcellular component is regulated by potassium transport on the apical membrane Na+-K+-2Cl cotransporter. The secretion of potassium begins in the early distal convoluted tubule of the nephron and progressively increases along the distal nephron into the cortical collecting duct. Most urinary K+ can be accounted for by electrogenic K+ secretion mediated by principal cells in the initial collecting duct and the cortical collecting duct. An electroneutral K+ and Cl cotransport mechanism is also present on the apical surface of the distal nephron. Under conditions of potassium deficiency, reabsorption of the cation occurs in the collecting duct. This process is regulated by the upregulation in the apically located H+-K+-ATPase on -intercalated cells.
Potassium is the most abundant cation (approximately 150 to 160 mEq per liter) within human cells. Intracellular sodium content is relatively low. In the extracellular fluid, sodium predominates and the potassium content is low (3.5 to 5 mEq per liter). A membrane-bound enzyme, sodium-potassiumactivated adenosinetriphosphatase (Na +K +ATPase), actively transports or pumps sodium out and potassium into cells to maintain the concentration gradients. The intracellular to extracellular potassium gradients are necessary for nerve impulse signaling in such specialized tissues as the heart, brain, and skeletal muscle, and for the maintenance of physiologic renal function and maintenance of acid-base balance. High intracellular potassium concentrations are necessary for numerous cellular metabolic processes. Intracellular K+ serves as a reservoir to limit the fall in extracellular potassium concentrations occurring under pathologic conditions with loss of potassium from the body.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 28845
Submission : 2014-11-17
Status : Active
Type : II



Date of Issue : 2022-06-08
Valid Till : 2025-07-07
Written Confirmation Number : WC-0104
Address of the Firm :


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Market Place

Reply
29 Sep 2018

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

CAS Number : 299-27-4
Quantity Per Vial :
Price ($) : 230
Catalog Number : 1550001
Current Lot : R03620
Previous Lot : I1M103 (30-JUN-2017)
NDC Code :

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?