
Synopsis
Synopsis
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
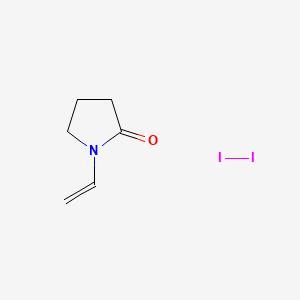

1. Alphadine
2. Alphadines
3. Betadine
4. Betadines
5. Betaisodona
6. Disadine
7. Disadines
8. Isodine
9. Isodines
10. Pharmadine
11. Pharmadines
12. Polyvinylpyrrolidone Iodine
13. Polyvinylpyrrolidone Iodines
14. Povidone-iodine
15. Povidone-iodines
16. Providine
17. Providines
18. Pvp Iodine
19. Pvp-i
20. Pvp-iodine
21. Pvp-iodines
1. 25655-41-8
2. Povidone-iodine
3. Betadine
4. Isodine
5. Pvp Iodine
6. Pvp-iodine
7. Pvp-i
8. Isobetadyne
9. Bridine
10. Disphex
11. Povadyne
12. Ultradine
13. Efo-dine
14. Iodopoly(vinyl Pyrrolidinone)
15. 1-ethenylpyrrolidin-2-one;molecular Iodine
16. Nsc26245
17. 2-pyrrolidinone, 1-ethenyl-, Homopolymer, Compd. With Iodineother Ca Index Names:iodine, Compd. With 1-ethenyl-2-pyrrolidinone Homopolymer
18. Iodine-poly(vinylpyrrolidinone)
19. Poly(vinylpyrrolidinone) Iodide
20. Iodinated Poly(vinylpyrrolidone)
21. Poly(vinylpyrrolidone)-iodine Adduct
22. Poly(vinylpyrrolidinone)-iodine Complex
23. Poly(vinylpyrrolidone) - Iodine Complex
24. Polyvinylpyrrolidone Compound With Iodine
25. Povidone.iodine
26. 1-ethenyl-2-pyrrolidinone Homopolymer Compound With Iodine
27. Molecular Iodine; 1-vinylpyrrolidin-2-one
28. 1-ethenylpyrrolidin-2-one; Molecular Iodine
29. Povidone Iodine-ip, 9-12%
30. Schembl1652685
31. Bcp28568
32. Nsc28655
33. Mfcd00084483
34. Nsc-26245
35. Nsc-28655
36. Akos015898248
37. Polyvinylpyrrolidone-iodinecomplex
38. 1-ethenyl-2-pyrrolidinone; Molecular Iodine
39. Ft-0655804
40. 2-pyrrolidinone, Polymers, Compd. With Iodine
41. A16118
42. 2-pyrrolidinone, Homopolymer, Compd. With Iodine
43. A817952
44. Q241516
45. 1-vinylpyrrolidin-2-one Compound With Diiodine (1:1)
46. Poly[1-(2-oxo-1-pyrrolidinyl)ethylene]iodine Complex
47. Povidone (iodinated), European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 364.95 g/mol |
|---|---|
| Molecular Formula | C6H9I2NO |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 364.87736 g/mol |
| Monoisotopic Mass | 364.87736 g/mol |
| Topological Polar Surface Area | 20.3 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Betadine |
| PubMed Health | Povidone Iodine (Into the eye) |
| Drug Classes | Antiseptic |
| Drug Label | Povidone-Iodine is a broad-spectrum microbicide with the chemical formulas:2-pyrrolidinone, 1- ethenyl-, homopolymer, compound with iodine; 1-vinyl-2-pyrrolidinone polymer, compound with iodine. The structural formula is as follows:BETADINE 5% Ster... |
| Active Ingredient | Povidone-iodine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 5% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Povidone iodine |
| PubMed Health | Povidone Iodine (Into the eye) |
| Drug Classes | Antiseptic |
| Drug Label | Other InformationStore below 98F/37C avoid direct heat and sunlight... |
| Active Ingredient | Povidone-iodine |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 1% |
| Market Status | Over the Counter |
| Company | Allegiance Hlthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Betadine |
| PubMed Health | Povidone Iodine (Into the eye) |
| Drug Classes | Antiseptic |
| Drug Label | Povidone-Iodine is a broad-spectrum microbicide with the chemical formulas:2-pyrrolidinone, 1- ethenyl-, homopolymer, compound with iodine; 1-vinyl-2-pyrrolidinone polymer, compound with iodine. The structural formula is as follows:BETADINE 5% Ster... |
| Active Ingredient | Povidone-iodine |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 5% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Povidone iodine |
| PubMed Health | Povidone Iodine (Into the eye) |
| Drug Classes | Antiseptic |
| Drug Label | Other InformationStore below 98F/37C avoid direct heat and sunlight... |
| Active Ingredient | Povidone-iodine |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 1% |
| Market Status | Over the Counter |
| Company | Allegiance Hlthcare |
Anti-Infective Agents, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 2014)
Povidone-iodine is an iodophore that is used as a disinfectant and antiseptic mainly for the treatment of contaminated wounds and pre-operative preparation of the skin and mucous membranes as well as for disinfection of equipment.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 1659
BETADINE 5% Sterile Ophthalmic Prep Solution for the eye is indicated for prepping of the periocular region (lids, brow, and cheek) and irrigation of the ocular surface (cornea, conjunctiva, and palpebral fornices).
NIH; DailyMed. Current Medication Information for BETADINE- povidone-iodine solution (Revised: July 2011). Available from, as of October 14, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b026de1b-9949-4557-ac49-c7b0038c24cd
No studies are available in patients with thyroid disorders; therefore, caution is advised in using BETADINE 5% Sterile Ophthalmic Prep Solution in these patients due to the possibility of iodine absorption.
NIH; DailyMed. Current Medication Information for BETADINE- povidone-iodine solution (Revised: July 2011). Available from, as of October 14, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b026de1b-9949-4557-ac49-c7b0038c24cd
Because of the potential for serious adverse reactions in nursing infants from BETADINE 5% Sterile Ophthalmic Prep Solution, a decision should be made to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
NIH; DailyMed. Current Medication Information for BETADINE- povidone-iodine solution (Revised: July 2011). Available from, as of October 14, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b026de1b-9949-4557-ac49-c7b0038c24cd
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
NIH; DailyMed. Current Medication Information for BETADINE- povidone-iodine solution (Revised: July 2011). Available from, as of October 14, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b026de1b-9949-4557-ac49-c7b0038c24cd
For external use only. Not for intraocular injection or irrigation.
NIH; DailyMed. Current Medication Information for BETADINE- povidone-iodine solution (Revised: July 2011). Available from, as of October 14, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b026de1b-9949-4557-ac49-c7b0038c24cd
For more Drug Warnings (Complete) data for Povidone-iodine (9 total), please visit the HSDB record page.
For topical application in the treatment and prevention of infection in wounds.
Povidone iodine is a kind of iodine disinfectant which directly cause in vivo protein denaturation, precipitation of bacteria, and further resulting in the death of pathogenic microorganisms. Therefore, it is effective in disinfection and sterilization. It can kill viruses, bacteria, spores, fungi, and protozoa with low toxicity to human. Povidone-iodine aqueous solution has strong pharmacological activity against Staphylococcus aureus, Neisseria gonorrhoeae, Pseudomonas aeruginosa, syphilis, hepatitis B virus, HIV, and Trichomonas vaginalis. Povidone iodine gel is a gynecological topical semi-mobile colloidal agent made by povidone iodine and hydrophilic matrix. It is a system for maintaining its sustained release. Owing to the continuous release of free iodine, it can enable the skin and mucous membranes to maintain a certain effective concentration of iodine for killing bacteria. It is mainly used for gynecological vaginal infection. It exerted its effect through being miscible with vaginal secretions and further killing the inside pathogenic microorganisms, and thus blocking the spread of sexually transmitted diseases and invasion, as well as treating other infected vaginal diseases caused by other kinds of bacteria. Published reports on the in vitro antimicrobial efficacy of iodophors demonstrate that iodophors are bactericidal, mycobactericidal, and virucidal but can require prolonged contact times to kill certain fungi and bacterial spores. Three brands of povidone-iodine solution have demonstrated more rapid kill (seconds to minutes) of S. aureus and M. chelonae at a 1:100 dilution than did the stock solution. The virucidal activity of 75150 ppm available iodine was demonstrated against seven viruses. Other investigators have questioned the efficacy of iodophors against poliovirus in the presence of organic matter and rotavirus in distilled or tapwater. Manufacturers' data demonstrate that commercial iodophors are not sporicidal, but they are tuberculocidal, fungicidal, virucidal, and bactericidal at their recommended use-dilution.
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AG - Iodine products
D08AG02 - Povidone-iodine
D - Dermatologicals
D09 - Medicated dressings
D09A - Medicated dressings
D09AA - Medicated dressings with antiinfectives
D09AA09 - Povidone-iodine
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AC - Medicated shampoos
D11AC06 - Povidone-iodine
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AX - Other antiinfectives and antiseptics
G01AX11 - Povidone-iodine
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA15 - Povidone-iodine
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AX - Other antiinfectives
S01AX18 - Povidone-iodine
Absorption
Povidone-Iodine is intended for topical application and is not absorbed.
Route of Elimination
Povidone-Iodine is intended for topical application and is not eliminated.
Volume of Distribution
Povidone-Iodine is intended for topical application and has no volume of distribution.
Clearance
Povidone-Iodine is intended for topical application and is not eliminated.
Topical application of povidone iodine on the umbilical cord and normal intact skin of newborn infants resulted in significantly elevated plasma iodine levels. High iodine levels were also found in two neonates who had povidone iodine applied to denuded skin. No significant alteration in thyroid function was seen. The possible toxic manifestations of high plasma iodine levels are discussed.
PMID:909027 Pyati SP et al; J Pediatr. 91 (5): 825-8 (1977)
In 12 nonpregnant women, total iodine, protein-bound iodine, inorganic iodine, and thyroxine values were measured in serum before and 15, 30, 45 or 60 minutes after a two-minute vaginal disinfection with povidone-iodine (Betadine). Only 15 minutes after application, serum iodine levels were raised and remained significantly elevated 30, 45 and 60 minutes after disinfection. Serum concentrations of total iodine and inorganic iodine were increased up to fivefold to 15-fold, respectively; during the relative short period of observation, thyroxine levels were not altered.
PMID:7431610 Vorherr H et al; JAMA. 244 (23): 2628-9 (1980)
Povidone-Iodine is not absorbed or metabolized.
Povidone-Iodine is intended for topical application and is not eliminated.
Povidone-iodine is called iodophore which means povidone acts as a carrier of iodine. Iodine is considered as the active moiety that mediates microbicidal actions. When released from the complex, free iodine (I2) penetrates the cell wall of microorganisms quickly, and the lethal effects are believed to result from disruption of protein and nucleic acid structure and synthesis. While the full mechanism of action is not fully elucidated, iodine is thought to inhibit vital bacterial cellular mechanisms and structures, and oxidizes nucleotides fatty or amino acids in bacterial cell membranes. Additionally, free iodine disrupts the function of the cytosolic enzymes involved in the respiratory chain, causing them to become denatured and deactivated. _In vitro_ evidence suggests that iodine also counteracts inflammation elicited by both pathogens and the host response via multifactorial effects. In hosts, povidone-iodine was demonstrated to modulate the redox potential, inhibit the release of inflammatory mediators such as TNF- and -galactosidase, inhibit metalloproteinase production, and potentiate the healing signals from pro-inflammatory cytokines by activation of monocytes, T-lymphocytes, and macrophages, _in vitro_.
 Chynops Pharma is an ideal sourcing partner for high-quality APIs, advanced intermediates, speciality chemicals & excipients.
Chynops Pharma is an ideal sourcing partner for high-quality APIs, advanced intermediates, speciality chemicals & excipients.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33619
Submission : 2019-04-02
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29888
Submission : 2015-10-21
Status : Active
Type : II

Certificate Number : CEP 2022-428 - Rev 00
Issue Date : 2024-05-15
Type : Chemical
Substance Number : 1142
Status : Valid


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36110
Submission : 2021-07-14
Status : Active
Type : II

Certificate Number : R1-CEP 2016-046 - Rev 00
Issue Date : 2021-05-05
Type : Chemical
Substance Number : 685
Status : Valid


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22686
Submission : 2009-04-01
Status : Active
Type : II

Registration Number : 218MF10060
Registrant's Address : 1820 Delmar Blvd. , St. Louis, MO 63103, USA
Initial Date of Registration : 2006-01-27
Latest Date of Registration :

NDC Package Code : 44242-100
Start Marketing Date : 1981-04-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33462
Submission : 2018-12-28
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 183
Submission : 1952-02-29
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3017
Submission : 1977-07-22
Status : Active
Type : II

Registration Number : 217MF10985
Registrant's Address : 100 Park Avenue, Florham Park, New Jersey, 07932, United States of America
Initial Date of Registration : 2005-11-21
Latest Date of Registration :

NDC Package Code : 70177-0002
Start Marketing Date : 2010-01-11
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
23
PharmaCompass offers a list of Povidone-Iodine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Povidone-Iodine manufacturer or Povidone-Iodine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Povidone-Iodine manufacturer or Povidone-Iodine supplier.
PharmaCompass also assists you with knowing the Povidone-Iodine API Price utilized in the formulation of products. Povidone-Iodine API Price is not always fixed or binding as the Povidone-Iodine Price is obtained through a variety of data sources. The Povidone-Iodine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Povidone Iodine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Povidone Iodine, including repackagers and relabelers. The FDA regulates Povidone Iodine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Povidone Iodine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Povidone Iodine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Povidone Iodine supplier is an individual or a company that provides Povidone Iodine active pharmaceutical ingredient (API) or Povidone Iodine finished formulations upon request. The Povidone Iodine suppliers may include Povidone Iodine API manufacturers, exporters, distributors and traders.
click here to find a list of Povidone Iodine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Povidone Iodine DMF (Drug Master File) is a document detailing the whole manufacturing process of Povidone Iodine active pharmaceutical ingredient (API) in detail. Different forms of Povidone Iodine DMFs exist exist since differing nations have different regulations, such as Povidone Iodine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Povidone Iodine DMF submitted to regulatory agencies in the US is known as a USDMF. Povidone Iodine USDMF includes data on Povidone Iodine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Povidone Iodine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Povidone Iodine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Povidone Iodine Drug Master File in Japan (Povidone Iodine JDMF) empowers Povidone Iodine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Povidone Iodine JDMF during the approval evaluation for pharmaceutical products. At the time of Povidone Iodine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Povidone Iodine suppliers with JDMF on PharmaCompass.
A Povidone Iodine CEP of the European Pharmacopoeia monograph is often referred to as a Povidone Iodine Certificate of Suitability (COS). The purpose of a Povidone Iodine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Povidone Iodine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Povidone Iodine to their clients by showing that a Povidone Iodine CEP has been issued for it. The manufacturer submits a Povidone Iodine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Povidone Iodine CEP holder for the record. Additionally, the data presented in the Povidone Iodine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Povidone Iodine DMF.
A Povidone Iodine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Povidone Iodine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Povidone Iodine suppliers with CEP (COS) on PharmaCompass.
A Povidone Iodine written confirmation (Povidone Iodine WC) is an official document issued by a regulatory agency to a Povidone Iodine manufacturer, verifying that the manufacturing facility of a Povidone Iodine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Povidone Iodine APIs or Povidone Iodine finished pharmaceutical products to another nation, regulatory agencies frequently require a Povidone Iodine WC (written confirmation) as part of the regulatory process.
click here to find a list of Povidone Iodine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Povidone Iodine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Povidone Iodine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Povidone Iodine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Povidone Iodine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Povidone Iodine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Povidone Iodine suppliers with NDC on PharmaCompass.
Povidone Iodine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Povidone Iodine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Povidone Iodine GMP manufacturer or Povidone Iodine GMP API supplier for your needs.
A Povidone Iodine CoA (Certificate of Analysis) is a formal document that attests to Povidone Iodine's compliance with Povidone Iodine specifications and serves as a tool for batch-level quality control.
Povidone Iodine CoA mostly includes findings from lab analyses of a specific batch. For each Povidone Iodine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Povidone Iodine may be tested according to a variety of international standards, such as European Pharmacopoeia (Povidone Iodine EP), Povidone Iodine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Povidone Iodine USP).
