
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
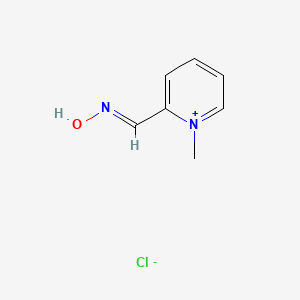

1. 1-methylpyridinium-2-aldoxime Ion
2. 2-formyl-1-methylpyridinium Chloride Oxime
3. 2-hydroxyiminomethyl-1-methylpyridinium
4. 2-hydroxyiminomethylpyridinium Methylmethanesulfonate
5. 2-pam
6. 2-pam Bromide
7. 2-pam Chloride
8. Contrathion
9. N-methylpyridinium 2-aldoxime Methylsulfate
10. Pralidoxime
11. Pralidoxime Bromide
12. Pralidoxime Chloride
13. Pralidoxime Fumarate (1:1)
14. Pralidoxime Iodide
15. Pralidoxime Lactate (1:1)
16. Pralidoxime Mesylate
17. Pralidoxime Methyl Sulfate
18. Pralidoxime Nitrate (1:1)
19. Pralidoxime Sulfate (1:1)
20. Pralidoxime Trichloroacetate
21. Pralidoxime, 14c-labeled
22. Protopam
23. Pyridine-2-aldoxime Methachloride
24. Pyridine-2-aldoxime Methiodide
25. Pyridine-2-aldoxime Methochloride
1. Pralidoxime Chloride
2. 2-pam Chloride
3. Protopam
4. 51-15-0
5. Combopen
6. Pralidoxine Chloride
7. Pyridine-2-aldoxime Methochloride
8. Pralidoxime Chloride [usan]
9. Pyridinium Aldoxime Methochloride
10. 2-pyridine Aldoxime Methyl Chloride
11. N-methylpyridine-2-aldoxime Chloride
12. 2-pyridinealdoxime Methochloride
13. N-methylpyridinium Chloride 2-aldoxime
14. N-methylpyridinium-2-aldoxime Chloride
15. 1-methyl-2-pyridinium Aldoxime Chloride
16. Pralidoxime (chloride)
17. 1-methyl-2-aldoximinopyridinium Chloride
18. 2-formyl-1-methylpyridinium Chloride Oxime
19. 2-formyl-n-methylpyridinium Oxime Chloride
20. 2-pam
21. 2-hydroxyiminomethyl-1-methylpyridinium Chloride
22. 14018-50-9
23. Pralidoxime Chloride [mi]
24. Nsc 164614
25. Nsc-164614
26. Pralidoxime Chloride [vandf]
27. Pralidoxime Chloride [mart.]
28. Mls000028727
29. Chebi:8355
30. Pyridinium, 2-((hydroxyimino)methyl)-1-methyl-, Chloride
31. Pralidoxime Chloride [usp-rs]
32. Pralidoxime Chloride [who-dd]
33. 38x7xs076h
34. Atnaa Component Pralidoxime Chloride
35. Pralidoxime Chloride [green Book]
36. Pralidoxime Chloride [orange Book]
37. Duodote Component Pralidoxime Chloride
38. Pralidoxime Chloride Component Of Atnaa
39. Pralidoxime Chloride Component Of Duodote
40. Smr000059189
41. 2-formyl-l-methylpyridinium Chloride Oxime
42. Dsstox_cid_3495
43. Pralidoxime Chloride (usp)
44. Pralidoxime Chloride [usp]
45. Dsstox_rid_77053
46. Dsstox_gsid_23495
47. 2-[(e)-(hydroxyimino)methyl]-1-methylpyridinium Chloride
48. Pyridinium, 2-((hydroxyimino)methyl)-1-methyl-, Chloride (e)-
49. (ne)-n-[(1-methylpyridin-1-ium-2-yl)methylidene]hydroxylamine;chloride
50. 2-pam (chloride)
51. 2-pyridine-aldoxime Chloride
52. Nsc164614
53. Einecs 200-080-9
54. N-metylpyridinium-2-aldoxime Chloride
55. 1-methyl-2-formylpyridinium Chloride Oxime
56. 2-pyridinealdoximemethochloride
57. Unii-38x7xs076h
58. Pralidoximchlorid
59. Pralidoxime Chloride [usan:usp]
60. Protopam (tn)
61. 2-(hydroxyiminomethyl)-1-methylpyridinium Chloride
62. Pralidoxime (inn)
63. Pam 2cl
64. Opera_id_228
65. Cas-51-15-0
66. Ncgc00016226-01
67. Pyridinium, Chloride, Oxime
68. Pyridinium, 2-formyl-1-methyl-, Chloride, Oxime
69. Schembl61419
70. Mls001076535
71. Mls002222315
72. Mls003876809
73. Schembl1650343
74. Dtxsid1023495
75. Hms1570n08
76. Hms2097n08
77. Hms3259i04
78. Hms3714n08
79. Pharmakon1600-01505449
80. Wln: T6kj A1 B1unq &g
81. 2-[(1e)-(hydroxyimino)methyl]-1-methylpyridin-1-ium Chloride
82. Hy-b1200
83. Tox21_110316
84. Mfcd00011981
85. Nsc759147
86. S4575
87. Akos001094374
88. Tox21_110316_1
89. Ccg-220943
90. Cs-4828
91. Nsc-759147
92. Ncgc00178286-05
93. As-13850
94. Bp164283
95. Pralidoxime Chloride [usp Monograph]
96. Cs-0369417
97. En300-50184
98. D00469
99. A828463
100. Q-201609
101. 2-((hydroxyimino)methyl)-1-methylpyridin-1-ium Chloride
102. (e)-2-((hydroxyimino)methyl)-1-methylpyridin-1-ium Chloride
103. Pyridinium, 2-[(hydroxyimino)methyl]-1-methyl-, Chloride (1:1)
104. (1-methyl-2-pyridylidene)methyl-oxo-ammonium Chloride;pralidoxime Chloride
105. Pralidoxime Chloride, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 172.61 g/mol |
|---|---|
| Molecular Formula | C7H9ClN2O |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 172.0403406 g/mol |
| Monoisotopic Mass | 172.0403406 g/mol |
| Topological Polar Surface Area | 36.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 125 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antidotes; Cholinesterase Reactivators
National Library of Medicine's Medical Subject Headings. Pralidoxime. Online file (MeSH, 2018). Available from, as of November 8, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Pralidoxime is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of November 8, 2018: https://clinicaltrials.gov/
Protopam chloride is indicated as an antidote: 1. In the treatment of poisoning due to those pesticides and chemicals (e.g., nerve agents) of the organophosphate class which have anticholinesterase activity and 2. In the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis. The principal indications for the use of Protopam chloride are muscle weakness and respiratory depression. In severe poisoning, respiratory depression may be due to muscle weakness. /Included in US product label/
NIH; DailyMed. Current Medication Information for Protopam Chloride (Pralidoxime Chloride Injection, Powder, Lyophilized, For Solution) (Updated: January 1, 2018). Available from, as of November 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2741d8fd-51c2-46be-880b-99f2b20a6137
Pralidoxime chloride is used concomitantly with atropine for the treatment of nerve agent poisoning in the context of chemical warfare or terrorism. Pralidoxime chloride must be administered within minutes to hours following exposure to nerve agents to be effective. /Included in US product label/
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3594
For more Therapeutic Uses (Complete) data for 2-PAM (8 total), please visit the HSDB record page.
IM administration of pralidoxime may produce mild pain at the injection site.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595
Rapid IV injection of pralidoxime has produced tachycardia, laryngospasm, muscle rigidity, and transient neuromuscular blockade; therefore, the drug should be administered slowly, preferably by IV infusion. IV administration of pralidoxime reportedly may also cause hypertension which is related to the dose and rate of infusion. Some clinicians recommend that the patient's blood pressure be monitored during pralidoxime therapy. For adults, IV administration of 5 mg of phentolamine mesylate reportedly quickly reverses pralidoxime-induced hypertension.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595
Although pralidoxime is generally well-tolerated, dizziness, blurred vision, diplopia and impaired accommodation, headache, drowsiness, nausea, tachycardia, hyperventilation, maculopapular rash, and muscular weakness have been reported following administration of the drug. However, it is difficult to differentiate the toxic effects produced by atropine or organophosphates from those of pralidoxime, and the condition of patients suffering from organophosphate intoxication will generally mask minor signs and symptoms reported in normal subjects who receive pralidoxime. When atropine and pralidoxime are used concomitantly, signs of atropinism may occur earlier than when atropine is used alone, especially if the total dose of atropine is large and administration of pralidoxime is delayed. Excitement, confusion, manic behavior, and muscle rigidity have been reported following recovery of consciousness, but these symptoms have also occurred in patients who were not treated with pralidoxime.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
NIH; DailyMed. Current Medication Information for Protopam Chloride (Pralidoxime Chloride Injection, Powder, Lyophilized, For Solution) (Updated: January 1, 2018). Available from, as of November 9, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2741d8fd-51c2-46be-880b-99f2b20a6137
For more Drug Warnings (Complete) data for 2-PAM (11 total), please visit the HSDB record page.
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Cholinesterase Reactivators
Drugs used to reverse the inactivation of cholinesterase caused by organophosphates or sulfonates. They are an important component of therapy in agricultural, industrial, and military poisonings by organophosphates and sulfonates. (See all compounds classified as Cholinesterase Reactivators.)
It is not known if pralidoxime crosses the human placenta to the embryo or fetus. Pralidoxime chloride is a quaternary ammonium compound, but the molecular weight of the free base (about 137) is low enough for passage across the placenta. The rapid elimination of the drug should mitigate this transfer.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Tenth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2015, p. 1140
The specific mechanism by which the renal tubule handles pralidoxime, a quaternary ammonium compound used to reactivate organophosphate-inhibited cholinesterase, has been studied using 22 subjects. Each subject was placed under certain conditions in the course of the study. All 22 received pralidoxime (5 mg/kg, IV, over a 2-min interval) under conditions of forced hydration and bed rest to serve as controls. Eight subjects received pralidoxime under conditions of forced hydration and bed rest, one time after 36 hr of ammonium chloride acidification, and another time after sodium bicarbonate alkalinization. Nine subjects received pralidoxime under forced dehydration and bed rest, 20-30 min after thiamine (200 mg total, IM), organic base. Eight received pralidoxime under forced hydration and bed rest simultaneously with p-aminohippurate (900 mg total, IV), organic acid. Four received pralidoxime under bed rest, after 8-12 hr of fasting, NPO. The drug is rapidly cleared from the plasma by renal tubular secretion. Reduction of pralidoxime clearance rates and prolongation of the biologic half-life after thiamine administration as compared to those after PAH administration suggest that pralidoxime is secreted as an organic base. Reduction of the excretion of pralidoxime under conditions of both urine alkalinization and urine acidification implicates an active reabsorption of pralidoxime not heretofore described.
Swartz RD, Sidell RR; Proc Soc Exp Biol Med 146: 419-424 (1974)
The pharmacokinetics of pralidoxime chloride (2-PAM) was studied in rats. Different groups of rats were given an intramuscular injection of 2-PAM at one of three doses (20, 40, or 80 mg/kg). This range of doses is used commonly in studies concerned with the efficacy of 2-PAM against poisoning by potent organophosphorus inhibitors of cholinesterase enzyme. Individual, sequential blood samples were collected during the course of the experiment. From these blood samples the plasma concentrations of 2-PAM were determined over time for each animal. Next the relationship of plasma concentration to time was expressed in terms of a standard pharmacokinetic model. Estimates of various pharmacokinetic parameters were calculated using an open, one-compartment model: volume of distribution (Vd), maximal plasma concentration (Cmax), elimination rate constant (k10), absorption rate constant (k01), area under the curve (AUC) and clearance (CL). Of the pharmacokinetic estimates, only Cmax and AUC were found to be statistically significant (p less than 0.0001) when compared across all the doses; these pharmacokinetic estimates were highly correlated with doses with r = 0.998 and r = 0.997, respectively. However, when AUC and Cmax were normalized by dividing through by dose, no significant differences were found in the transformed data. The results of this study in rat indicate that the pharmacokinetics of 2-PAM is linearly related to dose in a range employed in therapeutic studies of 2-PAM.
Green MD et al; Life Sci 39 (23): 2263-9 (1986)
BACKGROUND: Current therapies for organophosphate poisoning involve administration of oximes, such as pralidoxime (2-PAM), that reactivate the enzyme acetylcholinesterase. Studies in animal models have shown a low concentration in the brain following systemic injection. METHODS: To assess 2-PAM transport, we studied transwell permeability in three Madin-Darby canine kidney (MDCKII) cell lines and stem cell-derived human brain microvascular endothelial cells (BC1-hBMECs). To determine whether 2-PAM is a substrate for common brain efflux pumps, experiments were performed in the MDCKII-MDR1 cell line, transfected to overexpress the P-gp efflux pump, and the MDCKII-FLuc-ABCG2 cell line, transfected to overexpress the BCRP efflux pump. To determine how transcellular transport influences enzyme reactivation, we developed a modified transwell assay where the inhibited acetylcholinesterase enzyme, substrate, and reporter are introduced into the basolateral chamber. Enzymatic activity was inhibited using paraoxon and parathion. RESULTS: The permeability of 2-PAM is about 2 x 10(-6) cm/s in MDCK cells and about 1 x 10(-6) cm/s in BC1-hBMECs. Permeability is not influenced by pre-treatment with atropine. In addition, 2-PAM is not a substrate for the P-gp or BCRP efflux pumps. CONCLUSIONS: The low permeability explains poor brain penetration of 2-PAM and therefore the slow enzyme reactivation. This elucidates one of the reasons for the necessity of sustained intravascular (IV) infusion in response to organophosphate poisoning.
PMID:27396356 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4939658 Gallagher E et al; Fluids Barriers CNS 13 (1): 10 (2016)
For more Absorption, Distribution and Excretion (Complete) data for 2-PAM (10 total), please visit the HSDB record page.
Although the exact metabolic fate of pralidoxime has not been completely elucidated, the drug is believed to be metabolized in the liver. ... A recent study has suggested that active tubular secretion may be involved, although the specific mechanism has not been identified.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595
There is a trend towards increasing doses of pralidoxime to treat human organophosphate poisonings that may have relevance in subpopulations. Indeed, pralidoxime is eliminated unchanged by the renal route. This study assesses the effect of renal failure on the kinetics of pralidoxime in a rat model of acute renal failure induced by potassium dichromate administration. On the first day, Sprague-Dawley rats received subcutaneously potassium dichromate (study) or saline (control). Forty-eight hours post-injection, animals received pralidoxime methylsulfate (50 mg/kg of pralidoxime base) intramuscularly. Blood specimens were sampled during 180 min after the injection. Urine was collected daily during the 3 days of the study. Plasma pralidoxime concentrations were measured by liquid chromatography with electrochemical detection. There was a 2-fold increase in mean elimination half-life and a 2.5-fold increase in mean area under the curve in the study compared to the control group. The mean total body clearance was halved in the study compared to the control group. Our study showed acute renal failure does not modify the distribution of pralidoxime but significantly alters its elimination from plasma. These results suggest that dosages of pralidoxime should be adjusted in organophosphate-poisoned humans with renal failure when using high dosage regimen of pralidoxime.
Kayouka M et al; Toxicol Lett 184 (1): 61-6 (2009)
The half-life of pralidoxime in patients with normal renal function varies and has been reported to range from 0.8-2.7 hours.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595
Other reported pharmacologic effects of pralidoxime include depolarization at the neuromuscular junction, anticholinergic action, mild inhibition of cholinesterase, sympathomimetic effects, potentiation of the depressor action of acetylcholine in nonatropinized animals, and potentiation of the pressor action of acetylcholine in atropinized animals. However, the contribution of these effects to the therapeutic action of the drug has not been established.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595
The principal pharmacologic effect of pralidoxime is reactivation of cholinesterase which has been recently inactivated by phosphorylation as the result of exposure to certain organophosphates. Pralidoxime removes the phosphoryl group from the active site of the inhibited enzyme by nucleophilic attack, regenerating active cholinesterase and forming an oxime complex. Pralidoxime also detoxifies certain organophosphates by direct chemical reaction and probably also reacts directly with cholinesterase to protect it from inhibition. Pralidoxime must be administered before aging of the inhibited enzyme occurs; after aging is completed, phosphorylated cholinesterase cannot be reactivated, and newly synthesized cholinesterase must replace the inhibited enzyme. Pralidoxime is not equally antagonistic to all anticholinesterases, partly because the time period required for aging of the inhibited enzyme varies and depends on the specific organophosphate bound to the cholinesterase.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 3595

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
94
PharmaCompass offers a list of Pralidoxime Chloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pralidoxime Chloride manufacturer or Pralidoxime Chloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pralidoxime Chloride manufacturer or Pralidoxime Chloride supplier.
PharmaCompass also assists you with knowing the Pralidoxime Chloride API Price utilized in the formulation of products. Pralidoxime Chloride API Price is not always fixed or binding as the Pralidoxime Chloride Price is obtained through a variety of data sources. The Pralidoxime Chloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pralidoxime Chloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pralidoxime Chloride, including repackagers and relabelers. The FDA regulates Pralidoxime Chloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pralidoxime Chloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pralidoxime Chloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pralidoxime Chloride supplier is an individual or a company that provides Pralidoxime Chloride active pharmaceutical ingredient (API) or Pralidoxime Chloride finished formulations upon request. The Pralidoxime Chloride suppliers may include Pralidoxime Chloride API manufacturers, exporters, distributors and traders.
click here to find a list of Pralidoxime Chloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pralidoxime Chloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Pralidoxime Chloride active pharmaceutical ingredient (API) in detail. Different forms of Pralidoxime Chloride DMFs exist exist since differing nations have different regulations, such as Pralidoxime Chloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pralidoxime Chloride DMF submitted to regulatory agencies in the US is known as a USDMF. Pralidoxime Chloride USDMF includes data on Pralidoxime Chloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pralidoxime Chloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pralidoxime Chloride suppliers with USDMF on PharmaCompass.
A Pralidoxime Chloride written confirmation (Pralidoxime Chloride WC) is an official document issued by a regulatory agency to a Pralidoxime Chloride manufacturer, verifying that the manufacturing facility of a Pralidoxime Chloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pralidoxime Chloride APIs or Pralidoxime Chloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Pralidoxime Chloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Pralidoxime Chloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pralidoxime Chloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pralidoxime Chloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pralidoxime Chloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pralidoxime Chloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pralidoxime Chloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pralidoxime Chloride suppliers with NDC on PharmaCompass.
Pralidoxime Chloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pralidoxime Chloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pralidoxime Chloride GMP manufacturer or Pralidoxime Chloride GMP API supplier for your needs.
A Pralidoxime Chloride CoA (Certificate of Analysis) is a formal document that attests to Pralidoxime Chloride's compliance with Pralidoxime Chloride specifications and serves as a tool for batch-level quality control.
Pralidoxime Chloride CoA mostly includes findings from lab analyses of a specific batch. For each Pralidoxime Chloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pralidoxime Chloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Pralidoxime Chloride EP), Pralidoxime Chloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pralidoxime Chloride USP).
