
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
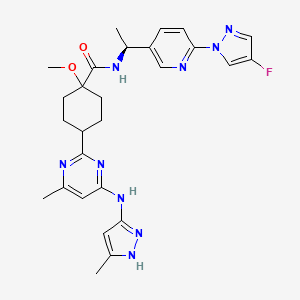

1. Blu-667
2. Cyclohexanecarboxamide, N-((1s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl)ethyl)-1-methoxy-4-(4-methyl-6-((5-methyl-1h-pyrazol-3-yl)amino)-2-pyrimidinyl)-, Trans-
3. Gavreto
4. Trans-n-((1s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl)ethyl)-1-methoxy-4-(4-methyl-6-((5-methyl-1h-pyrazol-3-yl)amino)-2-pyrimidinyl)cyclohexanecarboxamide
1. Blu-667
2. 2097132-94-8
3. Pralsetinib Free Base
4. Gavreto
5. Cis-pralsetinib
6. Blu667
7. Trans-pralsetinib
8. Pralsetinib [inn]
9. Pralsetinib [usan]
10. Blu123244
11. 1wpe73o1wv
12. 2097132-93-7
13. X581238
14. 2097132-94-8 (free Base)
15. Blu-123244
16. N-[(1s)-1-[6-(4-fluoropyrazol-1-yl)pyridin-3-yl]ethyl]-1-methoxy-4-[4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl]cyclohexane-1-carboxamide
17. X-581238
18. (cis)-n-((s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl)ethyl)-1-methoxy-4-(4-methyl-6-(5-methyl-1h-pyrazol-3-ylamino)pyrimidin-2-yl)cyclohexanecarboxamide
19. Cis-n-{(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl]ethyl}-1-methoxy-4-{4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl}cyclohexane-1-carboxamide
20. Cyclohexanecarboxamide, N-((1s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl)ethyl)-1-methoxy-4-(4-methyl-6-((5-methyl-1h-pyrazol-3-yl)amino)-2-pyrimidinyl)-, Cis-
21. Cyclohexanecarboxamide, N-[(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl]ethyl]-1-methoxy-4-[4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]-2-pyrimidinyl]-, Cis-
22. Q4j
23. Blu667blu667
24. Cis-blu-667
25. Pralsetinib (usan/inn)
26. Blu-667 (pralsetinib)
27. Unii-1wpe73o1wv
28. Pralsetinib [who-dd]
29. Chembl4582651
30. Schembl18789228
31. Schembl18789229
32. Schembl18806610
33. Gtpl10033
34. Bdbm435009
35. Bdbm435010
36. Dtxsid901336540
37. Amy16875
38. Ex-a1944
39. Ex-a3347
40. Nsc811429
41. S8716
42. Us10584114, Compound 129
43. Us10584114, Compound 130
44. Who 11004
45. Akos037648884
46. Hy-112301a
47. Nsc-811429
48. Ac-35657
49. Bs-15942
50. Hy-112301
51. Cs-0043448
52. Cs-0044766
53. D11712
54. Blu-667; Trans-n-{(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl]ethyl}-1-methoxy-4-{4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl}cyclohexane-1-carboxamide
55. Trans-n-{(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl]ethyl}-1-methoxy-4-{4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl}cyclohexane-1-carboxamide
| Molecular Weight | 533.6 g/mol |
|---|---|
| Molecular Formula | C27H32FN9O2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 533.26629946 g/mol |
| Monoisotopic Mass | 533.26629946 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 816 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of lung cancer (small cell and non-small cell lung cancer )
Gavreto is indicated as monotherapy for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced non-small cell lung cancer (NSCLC) not previously treated with a RET inhibitor.
Treatment of thyroid cancer
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L01XE
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX23 - Pralsetinib
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2024-06-26
Pay. Date : 2024-06-17
DMF Number : 39814
Submission : 2024-04-15
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF

GDUFA
DMF Review : Reviewed
Rev. Date : 2024-06-14
Pay. Date : 2024-05-20
DMF Number : 39659
Submission : 2024-05-24
Status : Active
Type : II

NDC Package Code : 73005-0010
Start Marketing Date : 2024-02-13
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


NDC Package Code : 50909-1905
Start Marketing Date : 2022-12-07
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (0.5kg/kg)
Marketing Category : DRUG FOR FURTHER PROCESSING


USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others

NDC Package Code : 81955-0018
Start Marketing Date : 2020-09-04
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2024-06-14
Pay. Date : 2024-05-20
DMF Number : 39659
Submission : 2024-05-24
Status : Active
Type : II

USDMF
GDUFA
DMF Review : Complete
Rev. Date : 2024-06-26
Pay. Date : 2024-06-17
DMF Number : 39814
Submission : 2024-04-15
Status : Active
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Beijing Sjar Technology Development Co., Ltd. founded in 2014, it is a high-tech enterprise which specialized in the research and development of active pharmaceutical ingredients a...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The partnership aims to assist in the commercialization of Rigel Pharmaceuticals' approved product, Gavreto (pralsetinib), which is indicated for RET fusion-positive non-small cell lung cancer.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Rigel Pharmaceuticals
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership June 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Rigel Pharmaceuticals
Deal Size : Undisclosed
Deal Type : Partnership
Optime Care Announces Enhanced Partnership with Rigel for GAVRETO® Patients
Details : The partnership aims to assist in the commercialization of Rigel Pharmaceuticals' approved product, Gavreto (pralsetinib), which is indicated for RET fusion-positive non-small cell lung cancer.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
June 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Rigel will able to commercialize the Gavreto (pralsetinib), which is an FDA-approved targeted therapy for RET fusion-positive metastatic non-small cell lung cancer.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Rigel Pharmaceuticals
Deal Size: $117.5 million Upfront Cash: Undisclosed
Deal Type: Acquisition February 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Rigel Pharmaceuticals
Deal Size : $117.5 million
Deal Type : Acquisition
Rigel Pharmaceuticals Acquires U.S. Rights to GAVRETO®
Details : Through the acquisition, Rigel will able to commercialize the Gavreto (pralsetinib), which is an FDA-approved targeted therapy for RET fusion-positive metastatic non-small cell lung cancer.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
February 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the terms of the agreement, Blueprint Medicines will regain global commercialization and development rights to Gavreto (pralsetinib), a once-daily oral targeted therapy, excluding Greater China.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: F. Hoffmann-La Roche
Deal Size: $1,702.0 million Upfront Cash: $775.0 million
Deal Type: Termination February 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : F. Hoffmann-La Roche
Deal Size : $1,702.0 million
Deal Type : Termination
Blueprint Medicines to Regain Global Rights to GAVRETO® (pralsetinib) from Roche
Details : Under the terms of the agreement, Blueprint Medicines will regain global commercialization and development rights to Gavreto (pralsetinib), a once-daily oral targeted therapy, excluding Greater China.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : $775.0 million
February 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Gavreto (pralsetinib) is a kinase inhibitor of wild-type RET and oncogenic RET fusions (CCDC6-RET) and mutations. It is approved for adult patients with locally advanced or metastatic RET fusion-positive NSCLC or thyroid cancer and advanced or metastatic RET-mutant MTC.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: BLU-667
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
CStone Announces the NDA Approval of GAVRETO® (pralsetinib) for the Treatment of Advanced RET Fus...
Details : Gavreto (pralsetinib) is a kinase inhibitor of wild-type RET and oncogenic RET fusions (CCDC6-RET) and mutations. It is approved for adult patients with locally advanced or metastatic RET fusion-positive NSCLC or thyroid cancer and advanced or metastatic...
Product Name : BLU-667
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GAVRETO is an important precision therapy that has been incredibly meaningful for patients with metastatic, RET fusion-positive non-small cell lung cancer who may have otherwise had limited options.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Royalty Pharma
Deal Size: $340.0 million Upfront Cash: $175.0 million
Deal Type: Collaboration June 30, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Royalty Pharma
Deal Size : $340.0 million
Deal Type : Collaboration
Details : GAVRETO is an important precision therapy that has been incredibly meaningful for patients with metastatic, RET fusion-positive non-small cell lung cancer who may have otherwise had limited options.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : $175.0 million
June 30, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Preclinical data have shown that Gavreto inhibits primary RET fusions and mutations that cause cancer in subsets of patients, as well as secondary RET mutations predicted to drive resistance to treatment.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
European Commission Approves Roche’s Gavreto (Pralsetinib) For the Treatment of Adults With RET ...
Details : Preclinical data have shown that Gavreto inhibits primary RET fusions and mutations that cause cancer in subsets of patients, as well as secondary RET mutations predicted to drive resistance to treatment.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
November 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Gavreto® (pralsetinib) is approved once-daily, oral targeted treatment designed to selectively target rearranged during transfection (RET) alterations. Gavreto showed robust and durable clinical responses in people with NSCLC with RET fusions.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Blueprint Medicines
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 17, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Blueprint Medicines
Deal Size : Inapplicable
Deal Type : Inapplicable
Roche Receives Positive CHMP Opinion For Gavreto® (pralsetinib) For the Treatment of Adults With ...
Details : Gavreto® (pralsetinib) is approved once-daily, oral targeted treatment designed to selectively target rearranged during transfection (RET) alterations. Gavreto showed robust and durable clinical responses in people with NSCLC with RET fusions.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 17, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Pralsetinib showed robust and durable anti-tumor activity and a well-tolerated safety profile in patients that enrolled at China sites who had advanced RET fusion-positive non-small cell lung cancer (NSCLC) previously treated with platinum-based chemotherapy.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: BLU-667
Study Phase: Phase I/ Phase IIProduct Type: Other Small Molecule
Sponsor: Blueprint Medicines
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 28, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Blueprint Medicines
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Pralsetinib showed robust and durable anti-tumor activity and a well-tolerated safety profile in patients that enrolled at China sites who had advanced RET fusion-positive non-small cell lung cancer (NSCLC) previously treated with platinum-based chemothe...
Product Name : BLU-667
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 28, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Developed by Blueprint Medicines, GAVRETO is a new therapy indicated for the treatment of adults with metastatic RET fusion-positive non-small cell lung cancer as detected by an FDA approved test. This agreement will continue Catalent’s involvement in the GAVRETO program.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Blueprint Medicines
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement November 23, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Blueprint Medicines
Deal Size : Undisclosed
Deal Type : Agreement
Details : Developed by Blueprint Medicines, GAVRETO is a new therapy indicated for the treatment of adults with metastatic RET fusion-positive non-small cell lung cancer as detected by an FDA approved test. This agreement will continue Catalent’s involvement in ...
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
November 23, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
GAVRETO demonstrated consistent clinical activity in patients across lines of therapy and regardless of RET mutation genotypes, including a high response rate in patients with gatekeeper mutations resistant to multi-kinase inhibitors.
Lead Product(s): Pralsetinib
Therapeutic Area: Oncology Brand Name: Gavreto
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Genentech
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 20, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Pralsetinib
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Genentech
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : GAVRETO demonstrated consistent clinical activity in patients across lines of therapy and regardless of RET mutation genotypes, including a high response rate in patients with gatekeeper mutations resistant to multi-kinase inhibitors.
Product Name : Gavreto
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 20, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : GAVRETO
Dosage Form : CAPSULE;ORAL
Dosage Strength : 100MG
Approval Date :
Application Number : 214701
RX/OTC/DISCN :
RLD :
TE Code :

Brand Name : GAVRETO
Dosage Form : CAPSULE;ORAL
Dosage Strength : 100MG
Approval Date : 2020-09-04
Application Number : 213721
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
91
PharmaCompass offers a list of Pralsetinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pralsetinib manufacturer or Pralsetinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pralsetinib manufacturer or Pralsetinib supplier.
PharmaCompass also assists you with knowing the Pralsetinib API Price utilized in the formulation of products. Pralsetinib API Price is not always fixed or binding as the Pralsetinib Price is obtained through a variety of data sources. The Pralsetinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pralsetinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pralsetinib, including repackagers and relabelers. The FDA regulates Pralsetinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pralsetinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pralsetinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pralsetinib supplier is an individual or a company that provides Pralsetinib active pharmaceutical ingredient (API) or Pralsetinib finished formulations upon request. The Pralsetinib suppliers may include Pralsetinib API manufacturers, exporters, distributors and traders.
click here to find a list of Pralsetinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pralsetinib DMF (Drug Master File) is a document detailing the whole manufacturing process of Pralsetinib active pharmaceutical ingredient (API) in detail. Different forms of Pralsetinib DMFs exist exist since differing nations have different regulations, such as Pralsetinib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pralsetinib DMF submitted to regulatory agencies in the US is known as a USDMF. Pralsetinib USDMF includes data on Pralsetinib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pralsetinib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pralsetinib suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pralsetinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pralsetinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pralsetinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pralsetinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pralsetinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pralsetinib suppliers with NDC on PharmaCompass.
Pralsetinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pralsetinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pralsetinib GMP manufacturer or Pralsetinib GMP API supplier for your needs.
A Pralsetinib CoA (Certificate of Analysis) is a formal document that attests to Pralsetinib's compliance with Pralsetinib specifications and serves as a tool for batch-level quality control.
Pralsetinib CoA mostly includes findings from lab analyses of a specific batch. For each Pralsetinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pralsetinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Pralsetinib EP), Pralsetinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pralsetinib USP).
