



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
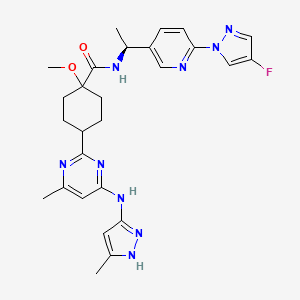

1. Blu-667
2. Cyclohexanecarboxamide, N-((1s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl)ethyl)-1-methoxy-4-(4-methyl-6-((5-methyl-1h-pyrazol-3-yl)amino)-2-pyrimidinyl)-, Trans-
3. Gavreto
4. Trans-n-((1s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl)ethyl)-1-methoxy-4-(4-methyl-6-((5-methyl-1h-pyrazol-3-yl)amino)-2-pyrimidinyl)cyclohexanecarboxamide
1. Blu-667
2. 2097132-94-8
3. Pralsetinib Free Base
4. Gavreto
5. Cis-pralsetinib
6. Blu667
7. Trans-pralsetinib
8. Pralsetinib [inn]
9. Pralsetinib [usan]
10. Blu123244
11. 1wpe73o1wv
12. 2097132-93-7
13. X581238
14. 2097132-94-8 (free Base)
15. Blu-123244
16. N-[(1s)-1-[6-(4-fluoropyrazol-1-yl)pyridin-3-yl]ethyl]-1-methoxy-4-[4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl]cyclohexane-1-carboxamide
17. X-581238
18. (cis)-n-((s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl)ethyl)-1-methoxy-4-(4-methyl-6-(5-methyl-1h-pyrazol-3-ylamino)pyrimidin-2-yl)cyclohexanecarboxamide
19. Cis-n-{(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl]ethyl}-1-methoxy-4-{4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl}cyclohexane-1-carboxamide
20. Cyclohexanecarboxamide, N-((1s)-1-(6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl)ethyl)-1-methoxy-4-(4-methyl-6-((5-methyl-1h-pyrazol-3-yl)amino)-2-pyrimidinyl)-, Cis-
21. Cyclohexanecarboxamide, N-[(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)-3-pyridinyl]ethyl]-1-methoxy-4-[4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]-2-pyrimidinyl]-, Cis-
22. Q4j
23. Blu667blu667
24. Cis-blu-667
25. Pralsetinib (usan/inn)
26. Blu-667 (pralsetinib)
27. Unii-1wpe73o1wv
28. Pralsetinib [who-dd]
29. Chembl4582651
30. Schembl18789228
31. Schembl18789229
32. Schembl18806610
33. Gtpl10033
34. Bdbm435009
35. Bdbm435010
36. Dtxsid901336540
37. Amy16875
38. Ex-a1944
39. Ex-a3347
40. Nsc811429
41. S8716
42. Us10584114, Compound 129
43. Us10584114, Compound 130
44. Who 11004
45. Akos037648884
46. Hy-112301a
47. Nsc-811429
48. Ac-35657
49. Bs-15942
50. Hy-112301
51. Cs-0043448
52. Cs-0044766
53. D11712
54. Blu-667; Trans-n-{(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl]ethyl}-1-methoxy-4-{4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl}cyclohexane-1-carboxamide
55. Trans-n-{(1s)-1-[6-(4-fluoro-1h-pyrazol-1-yl)pyridin-3-yl]ethyl}-1-methoxy-4-{4-methyl-6-[(5-methyl-1h-pyrazol-3-yl)amino]pyrimidin-2-yl}cyclohexane-1-carboxamide
| Molecular Weight | 533.6 g/mol |
|---|---|
| Molecular Formula | C27H32FN9O2 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 533.26629946 g/mol |
| Monoisotopic Mass | 533.26629946 g/mol |
| Topological Polar Surface Area | 136 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 816 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of lung cancer (small cell and non-small cell lung cancer )
Gavreto is indicated as monotherapy for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced non-small cell lung cancer (NSCLC) not previously treated with a RET inhibitor.
Treatment of thyroid cancer
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L01XE
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EX - Other protein kinase inhibitors
L01EX23 - Pralsetinib


