
Synopsis
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Australia

0
South Africa

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Batmen
2. Dermatop
3. Hoe 777
4. Hoe-777
5. Peitel
6. Prednisolone-17-ethylcarbonate-21-propionate
1. Dermatop
2. 73771-04-7
3. Hoe 777
4. Hoe-777
5. Dermatop E Emollient
6. S-770777
7. Mls002154121
8. V901lv1k7d
9. Las189961
10. S 77 0777
11. S-77 0777
12. Las-189961
13. Nsc-760042
14. Peitel
15. Las-41003 Component Prednicarbate
16. Unii-v901lv1k7d
17. S-77-0777
18. Prednicarbato
19. Prednicarbatum
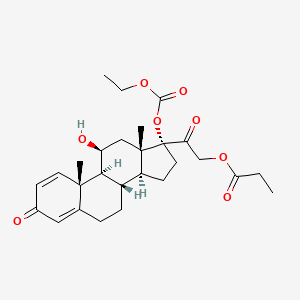
20. [2-[(8s,9s,10r,11s,13s,14s,17r)-17-ethoxycarbonyloxy-11-hydroxy-10,13-dimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Propanoate
21. Dermatop (tn)
22. Prednicarbate (usp/inn)
23. Prednicarbatum [inn-latin]
24. Prednicarbat
25. Prednicarbato [inn-spanish]
26. Ncgc00016926-01
27. Einecs 277-590-3
28. Prednicarbate [usan:usp:inn:ban]
29. Cas-73771-04-7
30. Prestwick0_001044
31. Prestwick1_001044
32. Prestwick2_001044
33. Prestwick3_001044
34. Prednicarbate [mi]
35. Prednicarbate [inn]
36. Schembl3941
37. Dsstox_cid_25502
38. Dsstox_rid_80919
39. Prednicarbate [usan]
40. Dsstox_gsid_45502
41. Bspbio_000968
42. Prednicarbate [vandf]
43. Prednicarbate [mart.]
44. S 770777
45. Spbio_002904
46. Prednicarbate [usp-rs]
47. Prednicarbate [who-dd]
48. Bpbio1_001066
49. Gtpl7605
50. Chembl1200386
51. Dtxsid9045502
52. Chebi:135791
53. Hms1571a10
54. Hms2098a10
55. Hms2230o11
56. Hms3715a10
57. Prednicarbate [orange Book]
58. Hy-b1365
59. Zinc3938652
60. Prednicarbate [ep Monograph]
61. Tox21_110687
62. Prednicarbate [usp Monograph]
63. Akos025402043
64. Prednicarbate For System Suitability A
65. Ac-3521
66. Ccg-221044
67. Db01130
68. Nsc 760042
69. 11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 17-(ethyl Carbonate) 21-propionate
70. Ncgc00179357-01
71. Ncgc00179357-05
72. Pregna-1,4-diene-3,20-dione, 17-((ethoxycarbonyl)oxy)-11-hydroxy-21-(1-oxopropoxy)-, (11beta)-
73. Smr001233428
74. Ab00514017
75. Cs-0013106
76. D05601
77. 771p047
78. Sr-01000841201
79. Q-101379
80. Q4376623
81. Sr-01000841201-2
82. Brd-k46137903-001-03-3
83. 11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione 17-(ethyl Carbonate) 21-propionate
84. Pregna-1,4-diene-3,20-dione, 17-((ethoxycarbonyl)oxy)-11-hydroxy-21-(1-oxopropoxy)-, (11.beta.)-
| Molecular Weight | 488.6 g/mol |
|---|---|
| Molecular Formula | C27H36O8 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 488.24101810 g/mol |
| Monoisotopic Mass | 488.24101810 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 982 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Dermatop |
| PubMed Health | Prednicarbate (Topical application route) |
| Drug Classes | Adrenal Glucocorticoid, Corticosteroid, Intermediate |
| Drug Label | DERMATOP Ointment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate. The topical corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and anti-pruritic... |
| Active Ingredient | Prednicarbate |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 2 of 6 | |
|---|---|
| Drug Name | Dermatop e emollient |
| PubMed Health | Prednicarbate (Topical application route) |
| Drug Classes | Adrenal Glucocorticoid, Corticosteroid, Intermediate |
| Drug Label | DERMATOP Ointment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate. The topical corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and anti-pruritic... |
| Active Ingredient | Prednicarbate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 3 of 6 | |
|---|---|
| Drug Name | Prednicarbate |
| Active Ingredient | Prednicarbate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 4 of 6 | |
|---|---|
| Drug Name | Dermatop |
| PubMed Health | Prednicarbate (Topical application route) |
| Drug Classes | Adrenal Glucocorticoid, Corticosteroid, Intermediate |
| Drug Label | DERMATOP Ointment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate. The topical corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and anti-pruritic... |
| Active Ingredient | Prednicarbate |
| Dosage Form | Ointment |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 5 of 6 | |
|---|---|
| Drug Name | Dermatop e emollient |
| PubMed Health | Prednicarbate (Topical application route) |
| Drug Classes | Adrenal Glucocorticoid, Corticosteroid, Intermediate |
| Drug Label | DERMATOP Ointment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate. The topical corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and anti-pruritic... |
| Active Ingredient | Prednicarbate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Valeant Bermuda |
| 6 of 6 | |
|---|---|
| Drug Name | Prednicarbate |
| Active Ingredient | Prednicarbate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Fougera Pharms |
For the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
FDA Label
Corticosteroids diffuse across cell membranes and complex with specific cytoplasmic receptors. These complexes then enter the cell nucleus, bind to DNA (chromatin), and stimulate transcription of messenger RNA (mRNA) and subsequent protein synthesis of various inhibitory enzymes responsible for the anti-inflammatory effects of topical corticosteroids. These anti-inflammatory effects include inhibition of early processes such as edema, fibrin deposition, capillary dilatation, movement of phagocytes into the area, and phagocytic activities. Later processes, such as capillary production, collagen deposition, and keloid formation also are inhibited by corticosteroids.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AC - Corticosteroids, potent (group iii)
D07AC18 - Prednicarbate
Absorption
Absorbed systemically across the stratum corneum.
Primarily in skin
In common with other topical corticosteroids, prednicarbate has anti-inflammatory, antipruritic, and vasoconstrictive properties. In general, the mechanism of the anti-inflammatory activity of topical steroids is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
32
PharmaCompass offers a list of Prednicarbate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Prednicarbate manufacturer or Prednicarbate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Prednicarbate manufacturer or Prednicarbate supplier.
PharmaCompass also assists you with knowing the Prednicarbate API Price utilized in the formulation of products. Prednicarbate API Price is not always fixed or binding as the Prednicarbate Price is obtained through a variety of data sources. The Prednicarbate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Prednicarbate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Prednicarbate, including repackagers and relabelers. The FDA regulates Prednicarbate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Prednicarbate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Prednicarbate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Prednicarbate supplier is an individual or a company that provides Prednicarbate active pharmaceutical ingredient (API) or Prednicarbate finished formulations upon request. The Prednicarbate suppliers may include Prednicarbate API manufacturers, exporters, distributors and traders.
click here to find a list of Prednicarbate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Prednicarbate DMF (Drug Master File) is a document detailing the whole manufacturing process of Prednicarbate active pharmaceutical ingredient (API) in detail. Different forms of Prednicarbate DMFs exist exist since differing nations have different regulations, such as Prednicarbate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Prednicarbate DMF submitted to regulatory agencies in the US is known as a USDMF. Prednicarbate USDMF includes data on Prednicarbate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Prednicarbate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Prednicarbate suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Prednicarbate Drug Master File in Korea (Prednicarbate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Prednicarbate. The MFDS reviews the Prednicarbate KDMF as part of the drug registration process and uses the information provided in the Prednicarbate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Prednicarbate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Prednicarbate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Prednicarbate suppliers with KDMF on PharmaCompass.
A Prednicarbate CEP of the European Pharmacopoeia monograph is often referred to as a Prednicarbate Certificate of Suitability (COS). The purpose of a Prednicarbate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Prednicarbate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Prednicarbate to their clients by showing that a Prednicarbate CEP has been issued for it. The manufacturer submits a Prednicarbate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Prednicarbate CEP holder for the record. Additionally, the data presented in the Prednicarbate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Prednicarbate DMF.
A Prednicarbate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Prednicarbate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Prednicarbate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Prednicarbate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Prednicarbate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Prednicarbate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Prednicarbate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Prednicarbate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Prednicarbate suppliers with NDC on PharmaCompass.
Prednicarbate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Prednicarbate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Prednicarbate GMP manufacturer or Prednicarbate GMP API supplier for your needs.
A Prednicarbate CoA (Certificate of Analysis) is a formal document that attests to Prednicarbate's compliance with Prednicarbate specifications and serves as a tool for batch-level quality control.
Prednicarbate CoA mostly includes findings from lab analyses of a specific batch. For each Prednicarbate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Prednicarbate may be tested according to a variety of international standards, such as European Pharmacopoeia (Prednicarbate EP), Prednicarbate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Prednicarbate USP).
