
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
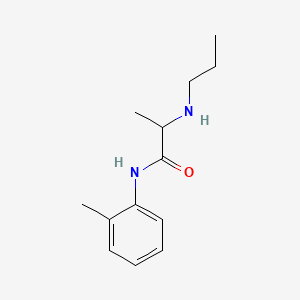

1. Citanest
2. Citanest Octapressin
3. Prilocaine Hydrochloride
4. Propitocaine
5. Xylonest
1. 721-50-6
2. Propitocaine
3. Citanest
4. Prilocainum
5. Prilocaine Base
6. N-(2-methylphenyl)-2-(propylamino)propanamide
7. Astra 1515
8. O-methyl-2-propylaminopropionanilide
9. Propanamide, N-(2-methylphenyl)-2-(propylamino)-
10. Prilocaina
11. 2-(propylamino)-o-propionotoluidide
12. O-propionotoluidide, 2-(propylamino)-
13. Astra 1512
14. O-methyl-alpha-propylaminopropionanilide
15. 2-methyl-alpha-propylaminopropionanilide
16. Alpha-n-propylamino-2-methylpropionanilide
17. Nsc 40027
18. Propitocaine (jan)
19. Chebi:8404
20. 2-(propylamino)-n-(o-tolyl)propanamide
21. 2-methyl-.alpha.-propylaminopropionanilide
22. Nsc-40027
23. Astra-1512
24. Astra-1515
25. O-propionotuluidide, 2-propylamino-
26. 046o35d44r
27. Prilocaine [usan]
28. Propitocaine [jan]
29. Prilocainum [inn-latin]
30. Prilocaina [inn-spanish]
31. L-67
32. (+/-)-prilocaine
33. Hsdb 3386
34. Prilocaine (usp/inn)
35. O-propionotoluidide, 2-propylamino-
36. Einecs 211-957-0
37. Brn 2108498
38. Prilotekal
39. Unii-046o35d44r
40. Prilocaine [usan:usp:inn:ban]
41. N-(2-methylphenyl)-n2-propylalaninamide
42. Spectrum_001649
43. L 67
44. Prilocaine [mi]
45. Prilocaine [inn]
46. N-(2-methylphenyl)-n(2)-propylalaninamide
47. N-(2-methylphenyl)-n~2~-propylalaninamide
48. Prestwick0_000199
49. Prestwick1_000199
50. Prestwick2_000199
51. Prestwick3_000199
52. Spectrum2_001549
53. Spectrum3_001052
54. Spectrum4_001192
55. Spectrum5_001175
56. (.+/-.)-prilocaine
57. Prilocaine [hsdb]
58. Prilocaine [vandf]
59. Prilocaine [mart.]
60. Chembl1194
61. Prilocaine [usp-rs]
62. Prilocaine [who-dd]
63. Lopac0_001005
64. Schembl25467
65. Bspbio_000157
66. Bspbio_002604
67. Kbiogr_001883
68. Kbioss_002129
69. Divk1c_000846
70. Spbio_001398
71. Spbio_002078
72. Bpbio1_000173
73. Gtpl7276
74. Dl-(+/-)-prilocaine
75. Emla Component Prilocaine
76. Dtxsid7031955
77. Prilocaine [orange Book]
78. Kbio1_000846
79. Kbio2_002129
80. Kbio2_004697
81. Kbio2_007265
82. Kbio3_001824
83. Prilocaine [ep Monograph]
84. Ninds_000846
85. Oraqix Component Prilocaine
86. Hms3604h09
87. Hms3651m13
88. Hms3884a04
89. Prilocaine [usp Monograph]
90. Act04759
91. Fortacin Component Prilocaine
92. Hy-b0137
93. Nsc40027
94. Prilocaine Component Of Emla
95. Bdbm50225477
96. Mfcd00048681
97. S1619
98. Stl257086
99. 2-(propylamino)-n-o-tolylpropanamide
100. Prilocaine Component Of Oraqix
101. Akos015889404
102. Ac-2100
103. Ccg-205085
104. Cs-1929
105. Db00750
106. Prilocaine Component Of Fortacin
107. Sdccgsbi-0050978.p004
108. Idi1_000846
109. Ncgc00015860-02
110. Ncgc00015860-03
111. Ncgc00015860-15
112. Ncgc00162312-01
113. As-14851
114. O-methyl-.alpha.-propylaminopropionanilide
115. Sbi-0050978.p003
116. Db-055611
117. Ab00053665
118. Ft-0603506
119. Ft-0660568
120. Sw196783-3
121. .alpha.-n-propyl-amino-2-methylpropionanilide
122. 86p818
123. C07531
124. D00553
125. Ab00053665-12
126. Ab00053665_13
127. Ab00053665_14
128. A837435
129. N-(2-methylphenyl)-2-(propylamino)propanamide #
130. Q413598
131. N-(2-methylphenyl)-2-(propylamino)propanimidic Acid
132. Q-100809
133. Brd-a53952395-003-05-3
134. Brd-a53952395-003-15-2
135. (+/-)-n-(2-propylaminopropionyl)-2-toluidine
136. N-(2,6-dimethylphenyl)-2-(propylamino)propanamide
| Molecular Weight | 220.31 g/mol |
|---|---|
| Molecular Formula | C13H20N2O |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 5 |
| Exact Mass | 220.157563266 g/mol |
| Monoisotopic Mass | 220.157563266 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 218 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anesthetics, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
AN AGENT CHEMICALLY SIMILAR TO LIDOCAINE & MEPIVACAINE USED FOR LOCAL & REGIONAL-BLOCK ANESTHESIA. IN ONSET OF ACTION & EFFECTIVENESS 1-3% SOLN... EQUIVALENT TO LIDOCAINE & MEPIVACAINE IN 1%-2% CONCN. ITS DURATION OF ACTION IS INTERMEDIATE TO SHORTER-ACTING LIDOCAINE & LONGER-ACTING MEPIVACAINE. /PRILOCAINE HCL/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 994
PRILOCAINE HYDROCHLORIDE ... HAS BEEN EMPLOYED ... FOR SPINAL ANESTHESIA. /PRILOCAINE HCL/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 390
... ACT ON ANY PART OF THE NERVOUS SYSTEM & ON EVERY TYPE OF NERVE FIBER. /LOCAL ANESTHETICS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 331
For more Therapeutic Uses (Complete) data for PRILOCAINE (10 total), please visit the HSDB record page.
AS WITH OTHER LOCAL ANESTHETICS, PRILOCAINE HCL IS CONTRAINDICATED IN PRESENCE OF SHOCK, SEVERE CARDIOVASCULAR DISEASE, OR HEART BLOCK. /PRILOCAINE HCL/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 994
... SHOULD NOT BE ADMIN TO PT WITH IDIOPATHIC OR CONGENITAL METHEMOGLOBINEMIA, ANEMIA, OR CARDIAC OR VENTILATORY FAILURE WITH HYPOXIA; IT SHOULD BE USED WITH CAUTION FOR CONTINUOUS EPIDURAL ANESTHESIA SINCE THE METHEMOGLOBINEMIC EFFECT OF INDIVIDUAL DOSES IS ADDITIVE. /PRILOCAINE HCL/
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 170
IN PRESENCE OF HEMORRHAGE, SYMPATHETIC BLOCK PRODUCED BY EPIDURAL ANESTHESIA BECOMES EXTREMELY SIGNIFICANT & MAY RESULT IN RAPID & DELETERIOUS CIRCULATORY CHANGES. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 399
TWO OUTSTANDING DANGERS /OF CAUDAL ANESTHESIA/ ARE (1) INTRODUCING NEEDLE INTO VENOUS PLEXUS LINING SACRAL CANAL, WITH RESULTANT INTRAVASCULAR INJECTION OF DRUG, & (2) PENETRATING DURA, WITH DEVELOPMENT OF HIGH LEVEL OF SPINAL ANESTHESIA. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 399
For more Drug Warnings (Complete) data for PRILOCAINE (16 total), please visit the HSDB record page.
Used as a local anaesthetic and is often used in dentistry.
Prilocaine binds to the intracellular surface of sodium channels which blocks the subsequent influx of sodium into the cell. Action potential propagation and never function is, therefore, prevented. This block is reversible and when the drug diffuses away from the cell, sodium channel function is restored and nerve propagation returns.
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
N01BB04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01B - Anesthetics, local
N01BB - Amides
N01BB04 - Prilocaine
Route of Elimination
Prilocaine is metabolized in both the liver and the kidney and excreted via the kidney.
/CONCERNING/ HYDROLYSIS OF AMIDE BOND OF PRILOCAINE... PLASMA CONCN OF R-(-)-ENANTIOMER WERE FOUND TO BE LOWER THAN THOSE OF THE S-(+)-ENANTIOMER AFTER IV ADMIN TO CAT. IN VITRO STUDIES USING LIVER PREPN FROM VARIOUS MAMMALS CONFIRMED THE R-(-)-ISOMER TO BE HYDROLYZED @ MUCH HIGHER RATES THAN THE S-(+) FORM...
Testa, B. and P. Jenner. Drug Metabolism: Chemical & Biochemical Aspects. New York: Marcel Dekker, Inc., 1976., p. 241
THERE IS...MORE RAPID PRODN OF METHEMOGLOBINEMIA BY D-(-) FORM, CAUSED PRESUMABLY BY HIGHER BLOOD LEVELS OF HYDROLYSIS PRODUCT, O-TOLUIDINE.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 375
PRILOCAINE FETAL/MATERNAL CONCN RATIO: 1.0 /FROM TABLE/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 100
PRILOCAINE DOSE 0.2 G IV GAVE BLOOD CONCN 0.26 MG% @ 0.3 HR & 0.14 MG% @ 0.17 HR; DOSE 0.4 G IV GAVE BLOOD CONCN 0.15 MG% @ 0.12 HR & 0.08 MG% @ 0.33 HR; DOSE 0.4 G EPIDURAL BLOCK GAVE BLOOD CONCN 0.26 MG% @ 0.25 HR (PEAK); DOSE 0.4 G INTERCOSTAL BLOCK GAVE BLOOD CONCN 0.40 MG% @ 0.25 HR. /FROM TABLE/
Sunshine, I. (ed.). CRC Handbook of Analytical Toxicology. Cleveland: The Chemical Rubber Co., 1969., p. 370
The amide-linked local anesthetics are, in general, degraded by the hepatic endoplasmic reticulum, the initial reactions involving N-dealkylation and subsequent hydrolysis. However, with prilocaine, the initial step is hydrolytic, forming o-toluidine metabolites that can cause methemoglobinemia.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 338
BIOTRANSFORMATION OF PRILOCAINE...IN RATS GAVE O-TOLUIDINE & N-PROPYLALANINE.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 244
MEPIVACAINE-HCL (I-HCL) & PRILOCAINE-HCL (II-HCL) WERE INFUSED IV 250 MG INTO HEALTHY VOLUNTEERS. T/2 FOR I WAS GENERALLY LONGER THAN II; TOTAL BODY CLEARANCE II CONSISTENTLY GREATER THAN I. II CLEARANCE EXCEEDED NORMAL HEPATIC BLOOD FLOW: EXTRA-HEPATIC METAB SITE IS POSTULATED.
ARTHUR ET AL; BR J ANAESTH 51(6) 481 (1979)
Prilocaine acts on sodium channels on the neuronal cell membrane, limiting the spread of seizure activity and reducing seizure propagation. The antiarrhythmic actions are mediated through effects on sodium channels in Purkinje fibers.
... BLOCK CONDUCTION IN NERVE PERHAPS BY COMPETING WITH CA @ SOME SITE THAT CONTROLS PERMEABILITY OF MEMBRANE ... CA IS ALSO INVOLVED IN ACTION OF LOCAL ANESTHETICS ON SMOOTH MUSCLE ... & ON ADRENAL MEDULLA ... /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 380
... PREVENT THE GENERATION & THE CONDUCTION OF THE NERVE IMPULSE. THEIR PRIMARY SITE OF ACTION IS THE CELL MEMBRANE. ... BLOCK CONDUCTION BY DECREASING OR PREVENTING THE LARGE TRANSIENT INCREASE IN THE PERMEABILITY OF EXCITABLE MEMBRANES TO NA+ THAT NORMALLY IS PRODUCED BY A SLIGHT DEPOLARIZATION OF THE MEMBRANE. /LOCAL ANESTHETICS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 332
AS ANESTHETIC ACTION PROGRESSIVELY DEVELOPS IN A NERVE, THRESHOLD FOR ELECTRICAL EXCITABILITY INCR & SAFETY FACTOR FOR CONDUCTION DECR; WHEN THIS ACTION IS SUFFICIENTLY WELL-DEVELOPED, BLOCK OF CONDUCTION IS PRODUCED. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 380
.../2 POSSIBILITIES:/ ACHIEVE BLOCK BY INCR SURFACE PRESSURE OF LIPID LAYER THAT CONSTITUTES NERVE MEMBRANE...CLOSING PORES THROUGH WHICH IONS MOVE. ... /OR:/ AFFECT PERMEABILITY BY INCR DEGREE OF DISORDER OF MEMBRANE. /LOCAL ANESTHETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 382

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
67
PharmaCompass offers a list of Prilocaine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Prilocaine manufacturer or Prilocaine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Prilocaine manufacturer or Prilocaine supplier.
PharmaCompass also assists you with knowing the Prilocaine API Price utilized in the formulation of products. Prilocaine API Price is not always fixed or binding as the Prilocaine Price is obtained through a variety of data sources. The Prilocaine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Prilocaine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Prilocaine, including repackagers and relabelers. The FDA regulates Prilocaine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Prilocaine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Prilocaine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Prilocaine supplier is an individual or a company that provides Prilocaine active pharmaceutical ingredient (API) or Prilocaine finished formulations upon request. The Prilocaine suppliers may include Prilocaine API manufacturers, exporters, distributors and traders.
click here to find a list of Prilocaine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Prilocaine DMF (Drug Master File) is a document detailing the whole manufacturing process of Prilocaine active pharmaceutical ingredient (API) in detail. Different forms of Prilocaine DMFs exist exist since differing nations have different regulations, such as Prilocaine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Prilocaine DMF submitted to regulatory agencies in the US is known as a USDMF. Prilocaine USDMF includes data on Prilocaine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Prilocaine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Prilocaine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Prilocaine Drug Master File in Japan (Prilocaine JDMF) empowers Prilocaine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Prilocaine JDMF during the approval evaluation for pharmaceutical products. At the time of Prilocaine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Prilocaine suppliers with JDMF on PharmaCompass.
A Prilocaine CEP of the European Pharmacopoeia monograph is often referred to as a Prilocaine Certificate of Suitability (COS). The purpose of a Prilocaine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Prilocaine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Prilocaine to their clients by showing that a Prilocaine CEP has been issued for it. The manufacturer submits a Prilocaine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Prilocaine CEP holder for the record. Additionally, the data presented in the Prilocaine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Prilocaine DMF.
A Prilocaine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Prilocaine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Prilocaine suppliers with CEP (COS) on PharmaCompass.
A Prilocaine written confirmation (Prilocaine WC) is an official document issued by a regulatory agency to a Prilocaine manufacturer, verifying that the manufacturing facility of a Prilocaine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Prilocaine APIs or Prilocaine finished pharmaceutical products to another nation, regulatory agencies frequently require a Prilocaine WC (written confirmation) as part of the regulatory process.
click here to find a list of Prilocaine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Prilocaine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Prilocaine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Prilocaine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Prilocaine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Prilocaine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Prilocaine suppliers with NDC on PharmaCompass.
Prilocaine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Prilocaine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Prilocaine GMP manufacturer or Prilocaine GMP API supplier for your needs.
A Prilocaine CoA (Certificate of Analysis) is a formal document that attests to Prilocaine's compliance with Prilocaine specifications and serves as a tool for batch-level quality control.
Prilocaine CoA mostly includes findings from lab analyses of a specific batch. For each Prilocaine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Prilocaine may be tested according to a variety of international standards, such as European Pharmacopoeia (Prilocaine EP), Prilocaine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Prilocaine USP).
