
Synopsis
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
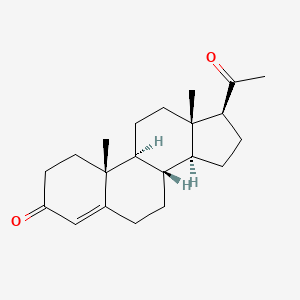

1. Pregnenedione
2. Progesterone, (13 Alpha,17 Alpha)-(+-)-isomer
3. Progesterone, (17 Alpha)-isomer
4. Progesterone, (9 Beta,10 Alpha)-isomer
1. 57-83-0
2. Pregn-4-ene-3,20-dione
3. Agolutin
4. Luteohormone
5. Crinone
6. 4-pregnene-3,20-dione
7. Utrogestan
8. Corpus Luteum Hormone
9. Progestin
10. Prometrium
11. Syngesterone
12. Luteol
13. Glanducorpin
14. Pregnenedione
15. Progestasert
16. Progesterol
17. Progesteronum
18. Corlutin
19. Cyclogest
20. Gesterol
21. Gestone
22. Gestormone
23. Progestone
24. Progestron
25. Hormoflaveine
26. Methylpregnone
27. Progestronol
28. Corlutina
29. Corluvite
30. Corporin
31. Flavolutan
32. Fologenon
33. Gynlutin
34. Gynolutone
35. Hormoluton
36. Lingusorbs
37. Lucorteum
38. Luteodyn
39. Luteogan
40. Luteopur
41. Luteosan
42. Luteostab
43. Luteovis
44. Lutociclina
45. Lutocyclin
46. Lutocylin
47. Lutoform
48. Lutromone
49. Membrettes
50. Nalutron
51. Piaponon
52. Primolut
53. Progekan
54. Progestosol
55. Prolidon
56. Proluton
57. Protormone
58. Syngestrets
59. Syntolutan
60. Gestron
61. Lutidon
62. Lutogyl
63. Lutren
64. Prolets
65. Lutex
66. Luteal Hormone
67. Lucorteum Sol
68. Bio-luton
69. Lutocyclin M
70. Lipo-lutin
71. Luteocrin Normale
72. Luteinique
73. Prochieve
74. Prolutone
75. Lutin
76. 17alpha-progesterone
77. Synovex S
78. Projestaject
79. Gynoluton
80. Gesterol 50
81. Percutacrine Luteinique
82. Gesterol 100
83. Cyclogesterin
84. Akrolutin
85. Endometrin
86. Prolutin
87. Pregnene-3,20-dione
88. (s)-progesterone
89. Colprosterone
90. Progesteron
91. Progestogel
92. Progeston
93. 3,20-pregnene-4
94. Gelbkoerperhormon
95. Crinone Progesterone Gel
96. Gestiron
97. Lugesteron
98. Progestol
99. Luteol (van)
100. Lutocuclin M
101. Nsc-9704
102. .beta.-progesterone
103. Percutacrine
104. (s)-4-pregnene-3,20-dione
105. Progeffik
106. Utrogest
107. Vitarrine
108. Luteum
109. Progesteronum [inn-latin]
110. Delta(4)-pregnene-3,20-dione
111. Progesterona [inn-spanish]
112. (s)-pregn-4-en-3,20-dione
113. Delta(sup 4)-pregnene-3,20-dione
114. Ccris 533
115. Prontogest
116. Estima
117. 17.alpha.-progesterone
118. Hsdb 3389
119. Progesterone [progestins]
120. Ai3-51682
121. Prometrium (tn)
122. Pregn-4-en-3,20-dione
123. Crinone (tn)
124. 6alpha-methylpregn-4-en-17alpha-ol-3,20-dione
125. Chebi:17026
126. .delta.4-pregnene-3,20-dione
127. 17alpha-hydroxy-6alpha-methylpregn-4-ene-3,20-dione
128. Progesterone, Micronized
129. Pregn-4-ene-3,20-dione, 17alpha-hydroxy-6alpha-methyl-
130. Component Of Cyclogesterin
131. Nsc-64377
132. 4-pregnen-3,20-dione
133. .delta.(sup4)-pregnene-3,20-dione
134. Chembl103
135. D4-pregnene-3,20-dione
136. Mls000028517
137. 4g7ds2q64y
138. (8s,9s,10r,13s,14s,17s)-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
139. Nsc9704
140. U 3672
141. Nsc64377
142. Ncgc00015785-04
143. Smr000058345
144. Dsstox_cid_2370
145. Dsstox_rid_76562
146. Dsstox_gsid_22370
147. (8s,9s,10r,13s,14s,17s)-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3h-cyclopenta[a]phenanthren-3-one
148. 6.alpha.-methylpregn-4-en-17.alpha.-ol-3,20-dione
149. 17.alpha.-hydroxy-6.alpha.-methylpregn-4-ene-3,20-dione
150. Progesterona
151. Progestan
152. Pregn-4-ene-3,20-dione, 17.alpha.-hydroxy-6.alpha.-methyl-
153. (8s,9s,10r,13s,14s,17s)-17-acetyl-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-3(2h)-one
154. Smr000653542
155. Progesterone (prometrium)
156. Wln: L E5 B666 Ov Mutj A1 E1 Fv1
157. Sr-01000000088
158. Sr-01000076054
159. Nsc 9704
160. Einecs 200-350-6
161. Nsc 64377
162. Pregn-4-ene-3, 17.alpha.-hydroxy-6.alpha.-methyl-
163. Bhr-100
164. Unii-4g7ds2q64y
165. Duraprogen
166. Progestrel
167. Lipolutin
168. Lutinus
169. Lutogynon
170. Progesterone??
171. Beta-progesterone
172. 1dbb
173. 17a-progesterone
174. Cas-57-83-0
175. Delta(sup4)-pregnene-3,20-dione
176. Progesterone [usp:inn:ban:jan]
177. Racemic Progesterone
178. Prestwick_411
179. Mfcd00003658
180. Cidr
181. Milprosa
182. Mpp22
183. 2aa6
184. 4bb2
185. Opera_id_292
186. Progesterone, >=99%
187. Prestwick0_000477
188. Prestwick1_000477
189. Prestwick2_000477
190. Prestwick3_000477
191. Spectrum5_002053
192. Progesterone [mi]
193. Cyclogesterin (salt/mix)
194. Progesterone [inn]
195. Progesterone [jan]
196. Bmse000482
197. Epitope Id:116051
198. Ec 200-350-6
199. Progesterone [hsdb]
200. Progesterone [inci]
201. Pregn-4-ne-3,20-ione
202. Schembl7671
203. Progesterone [vandf]
204. Bidd:pxr0094
205. Lopac0_000895
206. Bspbio_000614
207. Progesterone [mart.]
208. Mls000758277
209. Mls001074187
210. Mls001423982
211. Mls002222367
212. Bidd:er0547
213. P0130_sigma
214. Progesterone [usp-rs]
215. Progesterone [who-dd]
216. Progesterone [who-ip]
217. Spbio_002553
218. .delta.-pregnene-3,20-dione
219. Bdbm8903
220. Bpbio1_000676
221. Gtpl2377
222. Hydroxyprogesterone Caproic Acid
223. Dtxsid3022370
224. Progesterone (jp17/usp/inn)
225. Bhr-310
226. Eti-411
227. Progesterone [green Book]
228. 1a28
229. 1h60
230. Hms1569o16
231. Hms2051o05
232. Hms2090j07
233. Hms2096o16
234. Hms2230f23
235. Hms2233p11
236. Hms3262d12
237. Hms3713o16
238. Pregnene, 3,20-dione-delta^4-
239. Progesterone [orange Book]
240. Progesterone [ep Monograph]
241. Bcp22000
242. Bijuva Component Progesterone
243. Hy-n0437
244. Zinc4428529
245. Progesterone [usp Monograph]
246. Tox21_113157
247. Tox21_201792
248. Tox21_300307
249. Tox21_500895
250. Ac-700
251. Cmc_13406
252. Lmst02030159
253. Progesteronum [who-ip Latin]
254. S1705
255. Akos015894908
256. Progesterone; 4-pregnene-3,20-dione
257. Ccg-100766
258. Cs-1937
259. Db00396
260. Dr-2011
261. Fd12045
262. Lp00895
263. Nc00016
264. Progesterone Component Of Bijuva
265. Sdccgsbi-0050870.p002
266. Progesterone 1.0 Mg/ml In Acetonitrile
267. Progesterone 100 Microg/ml In Methanol
268. Ncgc00022185-03
269. Ncgc00022185-04
270. Ncgc00022185-05
271. Ncgc00022185-06
272. Ncgc00022185-07
273. Ncgc00022185-08
274. Ncgc00022185-09
275. Ncgc00022185-10
276. Ncgc00022185-11
277. Ncgc00022185-12
278. Ncgc00022185-14
279. Ncgc00022185-21
280. Ncgc00090798-01
281. Ncgc00090798-02
282. Ncgc00254120-01
283. Ncgc00259341-01
284. Ncgc00261580-01
285. (1s,10s,11s,14s,15s,2r)-14-acetyl-2,15-dimethyl-5-oxotetracyclo[8.7.0.0<2,7>.0 <11,15>]heptadec-6-ene
286. As-12660
287. Cpd000058345
288. Nci60_042166
289. Progesterone 1000 Microg/ml In Methanol
290. Fe-999913
291. Eu-0100895
292. P0478
293. (14beta,17alpha)-pregn-4-ene-3,20-dione
294. Progesterone, Meets Usp Testing Specifications
295. Progesterone, Vetec(tm) Reagent Grade, 98%
296. C00410
297. D00066
298. P 0130
299. Q26963
300. S00293
301. Progesterone, Vetranal(tm), Analytical Standard
302. Q-201624
303. Sr-01000000088-5
304. Sr-01000000088-6
305. Sr-01000076054-1
306. Sr-01000076054-4
307. Brd-k64994968-001-03-6
308. 32104fb6-bf81-4f6e-83c2-024deeaeb272
309. Progesterone, British Pharmacopoeia (bp) Reference Standard
310. Progesterone, Powder, Bioreagent, Suitable For Cell Culture
311. Progesterone, European Pharmacopoeia (ep) Reference Standard
312. Progesterone, Gamma-irradiated, Bioxtra, Suitable For Cell Culture
313. Progesterone, United States Pharmacopeia (usp) Reference Standard
314. Progesterone-water Soluble, Powder, Bioreagent, Suitable For Cell Culture
315. Progesterone For Peak Identification, European Pharmacopoeia (ep) Reference Standard
316. Progesterone For System Suitability, European Pharmacopoeia (ep) Reference Standard
317. Progesterone, Pharmaceutical Secondary Standard; Certified Reference Material
318. (1s,2r,10s,11s,14s,15s)-14-acetyl-2,15-dimethyltetracyclo[8.7.0.0;{2,7}.0;{11,15}]heptadec-6-en-5-one
319. 137940-28-4
320. 753497-20-0
321. Progesterone Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 314.5 g/mol |
|---|---|
| Molecular Formula | C21H30O2 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 314.224580195 g/mol |
| Monoisotopic Mass | 314.224580195 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 589 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Crinone |
| PubMed Health | Progesterone |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Active Ingredient | Progesterone |
| Dosage Form | Gel |
| Route | vaginal; Vaginal |
| Strength | 8%; 4% |
| Market Status | Prescription |
| Company | Watson Labs; Columbia Res Labs |
| 2 of 8 | |
|---|---|
| Drug Name | Endometrin |
| PubMed Health | Progesterone |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Drug Label | Endometrin (progesterone) Vaginal Insert contains micronized progesterone. Endometrin is supplied with polyethylene vaginal applicators.The active ingredient, progesterone, is present in 100 mg amount along with other excipients. The chemical name fo... |
| Active Ingredient | Progesterone |
| Dosage Form | Insert |
| Route | Vaginal |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Ferring |
| 3 of 8 | |
|---|---|
| Drug Name | Progesterone |
| PubMed Health | Progesterone |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Drug Label | PROMETRIUM (progesterone, USP) Capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C21H30O2. Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odo... |
| Active Ingredient | Progesterone |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 200mg; 50mg/ml; 100mg |
| Market Status | Prescription |
| Company | Sofgen Pharms; Hikma Farmaceutica; Watson Labs (utah); Banner Pharmacaps; Teva Pharms; Fresenius Kabi Usa; Luitpold |
| 4 of 8 | |
|---|---|
| Drug Name | Prometrium |
| PubMed Health | Progesterone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Drug Label | PROMETRIUM (progesterone, USP) Capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C21H30O2. Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odo... |
| Active Ingredient | Progesterone |
| Dosage Form | Capsule |
| Route | oral; Oral |
| Strength | 200mg; 300mg; 100mg |
| Market Status | Prescription |
| Company | Abbvie |
| 5 of 8 | |
|---|---|
| Drug Name | Crinone |
| PubMed Health | Progesterone |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Active Ingredient | Progesterone |
| Dosage Form | Gel |
| Route | vaginal; Vaginal |
| Strength | 8%; 4% |
| Market Status | Prescription |
| Company | Watson Labs; Columbia Res Labs |
| 6 of 8 | |
|---|---|
| Drug Name | Endometrin |
| PubMed Health | Progesterone |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Drug Label | Endometrin (progesterone) Vaginal Insert contains micronized progesterone. Endometrin is supplied with polyethylene vaginal applicators.The active ingredient, progesterone, is present in 100 mg amount along with other excipients. The chemical name fo... |
| Active Ingredient | Progesterone |
| Dosage Form | Insert |
| Route | Vaginal |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Ferring |
| 7 of 8 | |
|---|---|
| Drug Name | Progesterone |
| PubMed Health | Progesterone |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Drug Label | PROMETRIUM (progesterone, USP) Capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C21H30O2. Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odo... |
| Active Ingredient | Progesterone |
| Dosage Form | Capsule; Injectable |
| Route | Injection; Oral |
| Strength | 200mg; 50mg/ml; 100mg |
| Market Status | Prescription |
| Company | Sofgen Pharms; Hikma Farmaceutica; Watson Labs (utah); Banner Pharmacaps; Teva Pharms; Fresenius Kabi Usa; Luitpold |
| 8 of 8 | |
|---|---|
| Drug Name | Prometrium |
| PubMed Health | Progesterone (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Endometrial Hyperplasia Agent, Female Reproductive Agent |
| Drug Label | PROMETRIUM (progesterone, USP) Capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and a molecular formula of C21H30O2. Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odo... |
| Active Ingredient | Progesterone |
| Dosage Form | Capsule |
| Route | oral; Oral |
| Strength | 200mg; 300mg; 100mg |
| Market Status | Prescription |
| Company | Abbvie |
Progestins
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Prochieve 4% is indicated for the treatment of secondary amenorrhea. Prochieve 8% is indicated for use in women who have failed to respond to treatment with Prochieve 4%. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for PROCHIEVE (progesterone) gel (November 2009). Available from, as of March 1, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13486
Prochieve 8% is indicated for progesterone supplementation or replacement as part of an Assisted Reproductive Technology ("ART") treatment for infertile women with progesterone deficiency. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for PROCHIEVE (progesterone) gel (November 2009). Available from, as of March 1, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13486
Progesterone is used orally or intravaginally for the management of secondary amenorrhea.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3279
For more Therapeutic Uses (Complete) data for PROGESTERONE (9 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: CARDIOVASCULAR DISORDERS, BREAST CANCER and PROBABLE DEMENTIA FOR ESTROGEN PLUS PROGESTIN THERAPY. Cardiovascular Disorders and Probable Dementia: Estrogens plus progestin therapy should not be used for the prevention of cardiovascular disease or dementia. The Women's Health Initiative (WHI) estrogen plus progestin substudy reported increased risks of deep vein thrombosis, pulmonary embolism, stroke and myocardial infarction in postmenopausal women (50 to 79 years of age) during 5.6 years of treatment with daily oral conjugated estrogens (CE) (0.625 mg) combined with medroxyprogesterone acetate (MPA) (2.5 mg), relative to placebo. The WHI Memory Study (WHIMS) estrogen plus progestin ancillary study of the WHI reported an increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with daily CE (0.625 mg) combined with MPA (2.5 mg), relative to placebo. It is unknown whether this finding applies to younger postmenopausal women. Breast Cancer: The WHI estrogen plus progestin substudy also demonstrated an increased risk of invasive breast cancer. In the absence of comparable data, these risks should be assumed to be similar for other doses of CE and MPA, and other combinations and dosage forms of estrogens and progestins. Progestins with estrogens should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (Updated September 2013). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0224b5a6-25a3-44c9-b94f-90e2e064c164
Other doses of oral conjugated estrogens with medroxyprogesterone and other combinations and dosage forms of estrogens and progestins were not studied in the WHI clinical trials. In the absence of comparable data and product-specific studies, the relevance of the WHI findings to other products has not been established. Therefore, the risks should be assumed to be similar for all estrogen and progestin products. Because of these risks, estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (March 2008). Available from, as of February 23, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6971
Adverse effects reported in patients receiving oral progesterone include dizziness, breast pain, headache, abdominal pain, fatigue, viral infection, abdominal distention, musculoskeletal pain, emotional lability, irritability, and upper respiratory tract infection. Extreme dizziness and/or drowsiness, blurred vision, slurred speech, difficulty walking, loss of consciousness, vertigo, confusion, disorientation, and shortness of breath have been reported in a few women receiving the drug. Hypotension and syncope have occurred rarely in women receiving progesterone capsules.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3279
Adverse effects reported in patients receiving progesterone vaginal gel include breast pain/enlargement, somnolence, constipation, nausea, headache, and perineal pain.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3279
For more Drug Warnings (Complete) data for PROGESTERONE (19 total), please visit the HSDB record page.
**Gelatinized capsules** The gelatinized capsules are indicated for use in the prevention of endometrial hyperplasia in non-hysterectomized postmenopausal women who are receiving conjugated estrogens tablets. They are also indicated for use in secondary amenorrhea. **Vaginal gel** Progesterone gel (8%) is indicated as progesterone supplementation or replacement as part of an Assisted Reproductive Technology (ART) treatment for infertile women with progesterone deficiency. The lower concentration progesterone gel (4%) is used in the treatment of secondary amenorrhea, with the use of the 8% concentration if there is no therapeutic response to the 4% gel. **Vaginal insert** This form is indicated to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an Assisted Reproductive Technology (ART) treatment program for infertile women. **Injection (intramuscular)** This drug is indicated in amenorrhea and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer. **Tablets, contraceptive** The tablet form of progesterone in contraceptive formulations is indicated for the prevention of pregnancy.
FDA Label
Treatment of female infertility
Treatment of female infertility, Prevention of recurrent spontaneous abortion
Progesterone, depending on concentration and dosage form, and timing of exposure may have several pharmacodynamic effects. These actions, according, to various preparations, are listed below: General effects Progesterone is the main hormone of the corpus luteum and the placenta. It acts on the uterus by changing the proliferative phase to the secretory phase of the endometrium (inner mucous lining of the uterus). This hormone, stimulated by a hormone called _luteinizing hormone_ (LH) is the main hormone during the secretory phase to prepare the corpus luteum and the endometrium for implantation of a fertilized ovum. As the luteal phase concludes, the progesterone hormone sends negative feedback to the anterior pituitary gland in the brain to decrease FSH (follicle stimulating hormone) and LH (luteinizing hormone) levels. This prevents ovulation and maturation of oocytes (immature egg cells). The endometrium then prepares for pregnancy by increasing its vascularity (blood vessels) and stimulating mucous secretion. This process occurs by progesterone stimulating the endometrium to decrease endometrial proliferation, leading to a decreased uterine lining thickness, developing more complex uterine glands, collecting energy in the form of glycogen, and providing more uterine blood vessel surface area suitable for supporting a growing embryo. As opposed to cervical mucous changes observed during the proliferative phase and ovulation, progesterone decreases and thickens the cervical mucus, rendering it less elastic. This change occurs because the fertilization time period has passed, and a specific consistency of mucous amenable to sperm entry is no longer required. **Gelatinized capsules** Progesterone capsules are an oral dosage form of micronized progesterone which, chemically identical to progesterone of ovarian origin. Progesterone capsules have all the properties of endogenous progesterone with induction of a secretory phase endometrium with gestagenic, antiestrogenic, slightly antiandrogenic and anti-aldosterone effects. Progesterone opposes the effects of estrogen on the uterus, and is beneficial in women with unopposed estrogen exposure, which carries an increased risk of malignancy. **Vaginal gel and vaginal insert** The gel preparation mimics the effects of naturally occurring progesterone. In the presence of adequate levels of estrogen, progesterone converts a proliferative endometrium into secretory endometrium. This means that the endometrium changes from a growing and thickening stage into a subsequent preparation stage for pregnancy, which involves further preparatory changes. Progesterone is necessary for the development of decidual tissue (specialized tissue amenable to supporting a possible pregnancy). Progesterone is required to increase endometrial receptivity for the implantation of a fertilized embryo. Once an embryo is implanted, progesterone helps to maintain the pregnancy. **Injection (intramuscular)** Intramuscularly injected progesterone increases serum progesterone and aids in the prevention of endometrial tissue overgrowth due to unopposed estrogen (which leads to abnormal uterine bleeding and sometimes uterine cancer),. In the absence or deficiency of progesterone, the endometrium continually proliferates, eventually outgrowing its limited blood supply, shedding incompletely, and leading to abnormal and/or profuse bleeding as well as malignancy. **Tablets, contraceptive** Progesterone-only contraceptive tablets prevent conception by suppressing ovulation in about half of users, causing a thickening of cervical mucus to inhibit sperm movement, lowering the midcycle LH and FSH hormone peaks, slowing the movement of the ovum through the fallopian tubes, and causing secretory changes in the endometrium as described above.
Progestins
Compounds that interact with PROGESTERONE RECEPTORS in target tissues to bring about the effects similar to those of PROGESTERONE. Primary actions of progestins, including natural and synthetic steroids, are on the UTERUS and the MAMMARY GLAND in preparation for and in maintenance of PREGNANCY. (See all compounds classified as Progestins.)
G03DA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03D - Progestogens
G03DA - Pregnen (4) derivatives
G03DA04 - Progesterone
Absorption
**Oral micronized capsules** Following oral administration of progesterone in the micronized soft-gelatin capsule formulation, peak serum concentration was achieved in the first 3 hours. The absolute bioavailability of micronized progesterone is unknown at this time. In postmenopausal women, serum progesterone concentration increased in a dose-proportional and linear fashion after multiple doses of progesterone capsules, ranging from 100 mg/day to 300 mg/day. **IM administration** After intramuscular (IM) administration of 10 mg of progesterone in oil, the maximum plasma concentrations were achieved in about 8 hours post-injection and plasma concentrations stayed above baseline for approximately 24 hours post-injection. Injections of 10, 25, and 50 mg lead to geometric mean values for maximum plasma concentration (CMAX) of 7, 28, and 50 ng/mL, respectively. Progesterone administered by the intramuscular (IM) route avoids significant first-pass hepatic metabolism. As a result, endometrial tissue concentrations of progesterone achieved with IM administration are higher when compared with oral administration. Despite this, the highest concentrations of progesterone in endometrial tissue are reached with vaginal administration. **Note on oral contraceptive tablet absorption** Serum progestin levels peak about 2 hours after oral administration of progesterone-only contraceptive tablets, followed by rapid distribution and elimination. By 24 hours after drug administration, serum levels remain near the baseline, making efficacy dependent upon strict adherence to the dosing schedule. Large variations in serum progesterone levels occur among individuals. Progestin-only administration leads to lower steady-state serum progestin levels and a shorter elimination half-life than concurrent administration with estrogens.
Route of Elimination
Progesterone metabolites are excreted mainly by the kidneys. Urinary elimination is observed for 95% of patients in the form of glycuroconjugated metabolites, primarily 3 a, 5 pregnanediol (_pregnandiol_). The glucuronide and sulfate conjugates of pregnanediol and pregnanolone are excreted in the urine and bile. Progesterone metabolites, excreted in the bile, may undergo enterohepatic recycling or may be found excreted in the feces.
Volume of Distribution
When administered vaginally, progesterone is well absorbed by uterine endometrial tissue, and a small percentage is distributed into the systemic circulation. The amount of progesterone in the systemic circulation appears to be of minimal importance, especially when implantation, pregnancy, and live birth outcomes appear similar for intramuscular and vaginal administration of progesterone.
Clearance
**Apparent clearance** 1367 348 (50mg of progesterone administered by vaginal insert once daily). 106 15 L/h (50mg/mL IM injection once daily).
PROMETRIUM Capsules are an oral dosage form of micronized progesterone which is chemically identical to progesterone of ovarian origin. The oral bioavailability of progesterone is increased through micronization.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (March 2008). Available from, as of February 23, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6971
After oral administration of progesterone as a micronized soft-gelatin capsule formulation, maximum serum concentrations were attained within 3 hours. The absolute bioavailability of micronized progesterone is not known.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (March 2008). Available from, as of February 23, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6971
Serum progesterone concentrations appeared linear and dose proportional following multiple dose administration of PROMETRIUM Capsules 100 mg over the dose range 100 mg/day to 300 mg/day in postmenopausal women.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (March 2008). Available from, as of February 23, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6971
Although doses greater than 300 mg/day were not studied in females, serum concentrations from a study in male volunteers appeared linear and dose proportional between 100 mg/day and 400 mg/day. The pharmacokinetic parameters in male volunteers were generally consistent with those seen in postmenopausal women.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (March 2008). Available from, as of February 23, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6971
For more Absorption, Distribution and Excretion (Complete) data for PROGESTERONE (12 total), please visit the HSDB record page.
Progesterone is mainly metabolized by the liver. After oral administration, the major plasma metabolites found are 20 a hydroxy-4 a-prenolone and 5 a-dihydroprogesterone. Some progesterone metabolites are found excreted in the bile and these metabolites may be deconjugated and subsequently metabolized in the gut by reduction, dehydroxylation, and epimerization. The major plasma and urinary metabolites are comparable to those found during the physiological progesterone secretion of the corpus luteum.
Progesterone undergoes both biliary and renal elimination. Following an injection of labeled progesterone, 50-60% of the excretion of progesterone metabolites occurs via the kidney; approximately 10% occurs via the bile and feces, the second major excretory pathway.
US Natl Inst Health; DailyMed. Current Medication Information for PROCHIEVE (progesterone) gel (November 2009). Available from, as of March 1, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13486
Progesterone is metabolized primarily by the liver largely to pregnanediols and pregnanolones. Pregnanediols and pregnanolones are conjugated in the liver to glucuronide and sulfate metabolites. Progesterone metabolites which are excreted in the bile may be deconjugated and may be further metabolized in the gut via reduction, dehydroxylation, and epimerization.
US Natl Inst Health; DailyMed. Current Medication Information for Prometrium (progesterone) capsule (March 2008). Available from, as of February 23, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6971
The major urinary metabolite of oral progesterone is 5beta-pregnan-3alpha, 20alpha-diol glucuronide which is present in plasma in the conjugated form only. Plasma metabolites also include 5beta-pregnan-3alpha-ol-20-one (5beta-pregnanolone) and 5alpha-pregnan-3alpha-ol-20-one (5beta-pregnanolone).
US Natl Inst Health; DailyMed. Current Medication Information for PROCHIEVE (progesterone) gel (November 2009). Available from, as of March 1, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13486
The hormone is reduced to pregnanediol in the liver and conjugated with glucuronic acid, and then excreted mainly in urine.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3280
For more Metabolism/Metabolites (Complete) data for PROGESTERONE (9 total), please visit the HSDB record page.
Progesterone has known human metabolites that include 16beta-hydroxy-progesterone, 17alpha-hydroxy-progesterone, 21-hydroxy-progesterone, 2beta-hydroxy-progesterone, and 6beta-hydroxy-progesterone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Absorption half-life is approximately 25-50 hours and an elimination half-life of 5-20 minutes (progesterone gel). Progesterone, administered orally, has a short serum half-life (approximately 5 minutes). It is rapidly metabolized to _17-hydroxyprogesterone_ during its first pass through the liver.
Due to the sustained release properties of Prochieve, progesterone absorption is prolonged with an absorption half-life of approximately 25-50 hours, and an elimination half-life of 5-20 minutes. Therefore, the pharmacokinetics of Prochieve are rate-limited by absorption rather than by elimination.
US Natl Inst Health; DailyMed. Current Medication Information for PROCHIEVE (progesterone) gel (November 2009). Available from, as of March 1, 2011: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=13486
The elimination half life of progesterone is approximately 5 minutes ...
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1429
Progesterone has a short plasma half-life of several minutes.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3280
Progesterone binds and activates its nuclear receptor, _PR_, which plays an important part in the signaling of stimuli that maintain the endometrium during its preparation for pregnancy. Progesterone receptor (PR) is a member of the nuclear/steroid hormone receptor (SHR) family of ligand-dependent transcription factors that is expressed primarily in female reproductive tissue as well as the central nervous system. As a result of its binding its associated steroid hormone, progesterone, the progesterone receptor (PR) modulates the expression of genes that regulate the development, differentiation, and proliferation of target tissues. In humans, PR is found to be highly expressed in the stromal (connective tissue) cells during the secretory phase and during pregnancy. Progesterone may prevent pregnancy by changing the consistency of cervical mucus to be unfavorable for sperm penetration, and by inhibiting follicle-stimulating hormone (FSH), which normally causes ovulation. With perfect use, the first-year failure rate for progestin-only oral contraceptives is approximately 0.5%. The typical failure rate, however, is estimated to be approximately 5%, due to late or missed pills.
Progesterone is a progestinic hormone secreted mainly from the corpus luteum of the ovary during the latter half of the menstrual cycle. Progesterone is formed from steroid precursors in the ovary, testis, adrenal cortex, and placenta. Luteinizing hormone (LH) stimulates the synthesis and secretion of progesterone from the corpus luteum. Progesterone is necessary for nidation (implantation) of the ovum and for maintenance of pregnancy. Although the hormone is secreted mainly during the luteal phase of the menstrual cycle, small amounts of progesterone are also secreted during the follicular phase. High concentrations of the hormone are secreted during the latter part of pregnancy. Amounts comparable to those secreted in women during the follicular phase have been shown to be secreted in males.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3280
Progesterone shares the pharmacologic actions of the progestins. In women with adequate endogenous estrogen, progesterone transforms a proliferative endometrium into a secretory one. The abrupt decline in the secretion of progesterone at the end of the menstrual cycle is principally responsible for the onset of menstruation. Progesterone also stimulates the growth of mammary alveolar tissue and relaxes uterine smooth muscle. Progesterone has minimal estrogenic and androgenic activity.
American Society of Health System Pharmacists; AHFS Drug Information 2010. Bethesda, MD. (2010), p. 3280
Progesterone released during the luteal phase of the cycle decreases estrogen driven endometrial proliferation and leads to the development of a secretory endometrium ... . The abrupt decline in the release of progesterone from the corpus luteum at the end of the cycle is the main determinant of the onset of menstruation. If the duration of the luteal phase is artificially lengthened, either by sustaining luteal function or by treatment with progesterone, decidual changes in the endometrial stroma similar to those seen in early pregnancy can be induced. Under normal circumstances, estrogen antecedes and accompanies progesterone in its action upon the endometrium and is essential to the development of the normal menstrual pattern.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1428
Progesterone also increases the ventilatory response of the respiratory centers to carbon dioxide and leads to reduced arterial and alveolar PC02 in the luteal phase of the menstrual cycle and during pregnancy. Progesterone also may have depressant and hypnotic actions in the CNS, which may account for reports of drowsiness after hormone administration.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1428
For more Mechanism of Action (Complete) data for PROGESTERONE (12 total), please visit the HSDB record page.

239.9
> 200
219.7k
52.7M
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|---|---|---|
| INDIA | 1,33,521.71 | 190.5 | > 200 |
| INDIA | 9,773.94 | 1,004.7 | 11 - 50 |
| BELGIUM | 58,110.00 | 207.1 | 11 - 50 |
| INDIA | 242.39 | 1,090.9 | <10 |
| PAKISTAN | 418.00 | 608.5 | <10 |
| EGYPT | 1,321.00 | 134.8 | <10 |
| INDIA | 1,475.82 | 316.2 | <10 |
| INDIA | 5,425.00 | 223.3 | <10 |
| UNITED STATES | 39.16 | 4,508.2 | <10 |
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
29
PharmaCompass offers a list of Progesterone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Progesterone manufacturer or Progesterone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Progesterone manufacturer or Progesterone supplier.
PharmaCompass also assists you with knowing the Progesterone API Price utilized in the formulation of products. Progesterone API Price is not always fixed or binding as the Progesterone Price is obtained through a variety of data sources. The Progesterone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Progesterone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Progesterone, including repackagers and relabelers. The FDA regulates Progesterone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Progesterone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Progesterone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Progesterone supplier is an individual or a company that provides Progesterone active pharmaceutical ingredient (API) or Progesterone finished formulations upon request. The Progesterone suppliers may include Progesterone API manufacturers, exporters, distributors and traders.
click here to find a list of Progesterone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Progesterone DMF (Drug Master File) is a document detailing the whole manufacturing process of Progesterone active pharmaceutical ingredient (API) in detail. Different forms of Progesterone DMFs exist exist since differing nations have different regulations, such as Progesterone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Progesterone DMF submitted to regulatory agencies in the US is known as a USDMF. Progesterone USDMF includes data on Progesterone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Progesterone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Progesterone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Progesterone Drug Master File in Japan (Progesterone JDMF) empowers Progesterone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Progesterone JDMF during the approval evaluation for pharmaceutical products. At the time of Progesterone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Progesterone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Progesterone Drug Master File in Korea (Progesterone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Progesterone. The MFDS reviews the Progesterone KDMF as part of the drug registration process and uses the information provided in the Progesterone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Progesterone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Progesterone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Progesterone suppliers with KDMF on PharmaCompass.
A Progesterone CEP of the European Pharmacopoeia monograph is often referred to as a Progesterone Certificate of Suitability (COS). The purpose of a Progesterone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Progesterone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Progesterone to their clients by showing that a Progesterone CEP has been issued for it. The manufacturer submits a Progesterone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Progesterone CEP holder for the record. Additionally, the data presented in the Progesterone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Progesterone DMF.
A Progesterone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Progesterone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Progesterone suppliers with CEP (COS) on PharmaCompass.
A Progesterone written confirmation (Progesterone WC) is an official document issued by a regulatory agency to a Progesterone manufacturer, verifying that the manufacturing facility of a Progesterone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Progesterone APIs or Progesterone finished pharmaceutical products to another nation, regulatory agencies frequently require a Progesterone WC (written confirmation) as part of the regulatory process.
click here to find a list of Progesterone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Progesterone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Progesterone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Progesterone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Progesterone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Progesterone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Progesterone suppliers with NDC on PharmaCompass.
Progesterone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Progesterone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Progesterone GMP manufacturer or Progesterone GMP API supplier for your needs.
A Progesterone CoA (Certificate of Analysis) is a formal document that attests to Progesterone's compliance with Progesterone specifications and serves as a tool for batch-level quality control.
Progesterone CoA mostly includes findings from lab analyses of a specific batch. For each Progesterone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Progesterone may be tested according to a variety of international standards, such as European Pharmacopoeia (Progesterone EP), Progesterone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Progesterone USP).
