
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
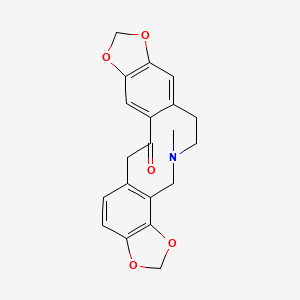

1. 4,6,7,14-tetrahydro-5-methyl-bis(1,3)benzodioxolo(4,5-c-5',6'-g)azecin-13(5h)-one
2. Fumarine
3. Protopine Hydrochloride
4. Protopine Mesylate
1. 130-86-9
2. Corydinine
3. Fumarine
4. Biflorine
5. Macleyine
6. Protopin
7. Hypercorine
8. 7,13a-secoberbin-13a-one, 7-methyl-2,3:9,10-bis(methylenedioxy)-
9. Chebi:16415
10. 7-methyl-2,3:9,10-bis(methylenedioxy)-7,13a-secoberbin-13a-one
11. 4,6,7,14-tetrahydro-5-methyl-bis(1,3)benzodioxolo(4,5-c-5',6'-g)azecin-13(5h)-one
12. 7-methyl-6,8,9,16-tetrahydrobis[1,3]benzodioxolo[4,5-c:5',6'-g]azecin-15(7h)-one
13. Chembl453019
14. Uiw569ht35
15. 7-methyl-6,7,8,9-tetrahydro-[1,3]dioxolo[4',5':5,6]benzo[1,2-c][1,3]dioxolo[5',4':4,5]benzo[1,2-g]azecin-15(16h)-one
16. Bis(1,3)benzodioxolo(4,5-c:5',6'-g)azecin-13(5h)-one, 4,6,7,14-tetrahydro-5-methyl-
17. Bis[1,3]benzodioxolo[4,5-c:5',6'-g]azecin-13(5h)-one, 4,6,7,14-tetrahydro-5-methyl-
18. 7-methyl-6,7,8,9-tetrahydro-[1,3]dioxolo[4',5':5,6]benzo[1,2-c][1,3]dioxolo[5',4':4,5]benzo[1,2-g]azecin-
19. Hsdb 3527
20. Einecs 204-999-6
21. Unii-uiw569ht35
22. Ai3-62909
23. Corydalis C
24. Alk-3
25. Protopine [mi]
26. Oprea1_718853
27. Oprea1_722246
28. Protopine, Analytical Standard
29. Schembl178013
30. Acon1_001550
31. Dtxsid90156282
32. Hms3428d22
33. Hms3885m10
34. Hy-n0793
35. Tnp00339
36. Bdbm50286643
37. Ccg-36008
38. Hsci1_000268
39. Mfcd00022322
40. Nsc781768
41. S3883
42. Stl561430
43. Zinc20111233
44. Akos000278163
45. Cs-4249
46. Nsc-781768
47. 4,6,7,14-tetrahydro-5-methyl-bis[1,3]benzodioxolo[4,5-c-5,6-g]azecin-13(5h)-one
48. Ncgc00017389-01
49. Ncgc00017389-02
50. Ncgc00142402-01
51. 7-methyl-4h,6h,8h,9h,12h,16h-1,3-dioxoleno[4''',5'''-3'',4'']benzo[1'',2''-8', 9']azecino[5',4'-2,1]benzo[4,5-d]1,3-dioxolan-15-one
52. Ac-34931
53. Bs-15163
54. Ft-0674127
55. N2275
56. P2611
57. A14808
58. C05189
59. Q390706
60. Sr-05000002290
61. Sr-05000002290-2
62. Brd-k83302049-001-01-6
63. Brd-k83302049-003-01-2
64. 2d13e16b-20f9-4670-ad3e-02ee26a53c9c
65. 7-methyl-2,3:9,10-bis[methylenebis(oxy)]-7,13a-secoberbin-13a-one
66. 4,6,7,14-tetrahydro-5-methylbis[1,3]benzodioxolo[4,5-c:5',6'-g]azecin-13(5h)-one
67. 7-methyl-6,8,9,16-tetrahydrodi[1,3]benzodioxolo[4,5-c:5,6-g]azecin-15(7h)-one #
68. 15-methyl-7,9,19,21-tetraoxa-15-azapentacyclo[15.7.0.0^{4,12}.0^{6,10}.0^{18,22}]tetracosa-1(24),4(12),5,10,17,22-hexaen-3-one
69. 15-methyl-7,9,19,21-tetraoxa-15-azapentacyclo[15.7.0.04,12.06,10.018,22]tetracosa-1(17),4,6(10),11,18(22),23-hexaen-3-one
| Molecular Weight | 353.4 g/mol |
|---|---|
| Molecular Formula | C20H19NO5 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 353.12632271 g/mol |
| Monoisotopic Mass | 353.12632271 g/mol |
| Topological Polar Surface Area | 57.2 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 542 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics, Opioid; Histamine H1 Antagonists; Platelet Aggregation Inhibitors
National Library of Medicine's Medical Subject Headings. Protopine Online file (MeSH, 2016). Available from, as of May 31, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/EXPL THER/ ... In the present study, we studied the anticancer proliferation and adhesion effects of five alkaloids which were isolated from Corydalis yanhusuo. MTT dose response curves, cell migration assay, cell invasion assay, as well as three types of cell adhesive assay were performed on MDA-MB-231 human breast cancer cells. The mechanism of the compounds on inhibiting heterotypic cell adhesion were further explored by determining the expression of epidermal growth factor receptor (EGFR), Intercellular adhesion molecule 1 (ICAM-1), alphav-integrin, beta1-integrin and beta5-integrin by western blotting assay. In five tested alkaloids, only protopine exhibited anti-adhesive and anti-invasion effects in MDA-MB-231 cells, which contributed to the anti-metastasis effect of Corydalis yanhusuo. The results showed that after treatment with protopine for 90 min, the expression of EGFR, ICAM-1, alphav-integrin, beta1-integrin and beta5-integrin were remarkably reduced. The present results suggest that protopine seems to inhibit the heterotypic cell adhesion between MDA-MB-231 cells, and human umbilical vein endothelial cells by changing the expression of adhesive factors.
PMID:25435628 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202652 He K, Gao JL; Afr J Tradit Complement Altern Med 11 (2): 415-24 (2014)
/EXPL THER/ ... In this study, we investigated whether protopine derived from Hypecoum erectum L could suppress lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (Raw 264.7 cells). Protopine was found to reduce nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E(2) (PGE(2)) production by LPS-stimulated Raw 264.7 cells, without a cytotoxic effect. Pre-treatment of Raw 264.7 cells with protopine reduced the production of pro-inflammatory cytokines. These inhibitory effects were caused by blocking phosphorylation of mitogen-activated protein kinases (MAP kinases) and also blocking activation of a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB).
PMID:22360889 Bae DS et al; BMB Rep 45 (2): 108-13 (2012)
/EXPL THER/ In this study, we investigated the anticancer effect of protopine on human hormone-refractory prostate cancer (HRPC) cells. Protopine exhibited an anti-proliferative effect by induction of tubulin polymerization and mitotic arrest, which ultimately led to apoptotic cell death. The data suggest that protopine increased the activity of cyclin-dependent kinase 1 (Cdk1)/cyclin B1 complex and that contributed to cell apoptosis by modulating mitochondria-mediated signaling pathways, such as Bcl-2 phosphorylation and Mcl-1 down-regulation. In conclusion, the data suggest that protopine is a novel microtubule stabilizer with anticancer activity in HRPC cells through apoptotic pathway by modulating Cdk1 activity and Bcl-2 family of proteins.
PMID:22033245 Chen CH et al; Cancer Lett 315 (1): 1-11 (2012)
For more Therapeutic Uses (Complete) data for PROTOPINE (12 total), please visit the HSDB record page.
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
Xiang-Fu-Si-Wu Decoction (XFSWD) has been widely used to treat primary dysmenorrhea in clinical practice for hundreds of years and shown great efficacy. One fraction of XFSWD, which was an elution product by macroporous adsorption resin from aqueous extract solution with 60% ethanol (XFSWE), showed great analgesic effect. The present study was conducted to investigate the possible pharmacokinetic and tissue distribution profiles of four major bioactive constituents (berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine) after oral administration of XFSWE in dysmenorrheal symptom rats, and to compare the difference between normal and dysmenorrheal symptom rats. Estradiol benzoate and oxytocin were used to produce dysmenorrheal symptom rat model. The experimental period was seven days. At the final day of experimental period, both normal and dysmenorrheal symptom rats were orally administrated with XFSWE, and then the blood and tissues samples were collected at different time points. Berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine in blood and tissue samples were determined by LC-MS/MS. Pharmacokinetic parameters were calculated from the plasma concentration-time data using non-compartmental methods. The differences of pharmacokinetic parameters among groups were tested by one-way analysis of variance (ANOVA). There were statistically significant differences (P<0.05) in Cmax, Tmax, AUC(0-t), AUC(0-infinity), MRT(0-t), MRT(0-infinity) and CL/F between normal and dysmenorrheal symptom rats that orally administered with same dosage of XFSWE. In tissue distribution study, the results showed that the overall trend was C(Spleen)>C(Liver)>C(Kidney)>C(Uterus)>C(Heart)>C(Lung)>C(Ovary)>C(Brain)>C(Thymus), C(M-60 min)>C(M-120 min)>C(M-30 min)>C(C-60 min)>C(C-120 min)>C(C-30 min). The contents of protopine in liver, spleen and uterus were more than that in other tissues of dysmenorrheal symptom rats. Compared to normal rats, partial contents of the compounds in dysmenorrheal symptom rats' tissues at different time points had significant difference (P<0.05). This study was the first report about pharmacokinetic and tissue distribution investigation in dysmenorrheal symptom animals. The results indicated that berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine have higher uptake and slower elimination in the rats with dysmenorrheal syndrome, which suggests that the rate and extent of drug metabolism were altered in dysmenorrheal syndrome rats. And the results also demonstrated that berberine, protopine and tetrahydropalmatine in normal and dysmenorrheal symptom rats had obvious differences in some organs and time points, suggesting that the blood flow and perfusion rate of the organ were altered in dysmenorrheal symptom animals.
PMID:24837303 Liu P et al; J Ethnopharmacol 154 (3): 696-703 (2014)
Eschscholtzia californica preparations are in use as phytopharmaceuticals and as herbal drugs. Studies are described on the metabolism and the toxicological analysis of the Eschscholtzia californica alkaloids californine and protopine in rat urine using gas chromatography-mass spectrometry. ... Protopine ... undergoes extensive demethylenation of the 2,3-methylenedioxy group followed by catechol-O-methylation. All phenolic hydroxy metabolites were found to be partly conjugated. The authors' systematic toxicological analysis procedure using full-scan gas chromatography-mass spectrometry after acid hydrolysis, liquid-liquid extraction and microwave-assisted acetylation allowed the detection of the main metabolites of californine and protopine in rat urine after a dose which should correspond to that of drug users. Therefore, use of Eschscholtzia californica preparations should also be detectable in human urine by the authors' systematic toxicological analysis procedure.
PMID:12726842 Paul LD, Maurer HH; J Chromatogr B Analyt Technol Biomed Life Sci 789 (1): 43-57 (2003)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
73
PharmaCompass offers a list of Protopine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Protopine manufacturer or Protopine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Protopine manufacturer or Protopine supplier.
PharmaCompass also assists you with knowing the Protopine API Price utilized in the formulation of products. Protopine API Price is not always fixed or binding as the Protopine Price is obtained through a variety of data sources. The Protopine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Protopine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Protopine, including repackagers and relabelers. The FDA regulates Protopine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Protopine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Protopine supplier is an individual or a company that provides Protopine active pharmaceutical ingredient (API) or Protopine finished formulations upon request. The Protopine suppliers may include Protopine API manufacturers, exporters, distributors and traders.
Protopine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Protopine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Protopine GMP manufacturer or Protopine GMP API supplier for your needs.
A Protopine CoA (Certificate of Analysis) is a formal document that attests to Protopine's compliance with Protopine specifications and serves as a tool for batch-level quality control.
Protopine CoA mostly includes findings from lab analyses of a specific batch. For each Protopine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Protopine may be tested according to a variety of international standards, such as European Pharmacopoeia (Protopine EP), Protopine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Protopine USP).
