
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
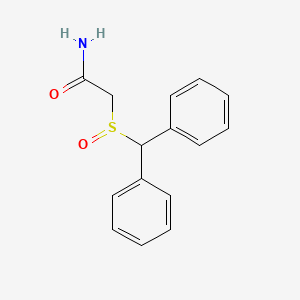

1. 2 Benzhydrylsulfinylacetamide
2. 2-((diphenylmethyl)sulfinyl)acetamide
3. 2-((r)-(diphenylmethyl)sulfinyl)acetamide
4. 2-(benzhydrylsulfinyl)acetamide
5. 2-benzhydrylsulfinylacetamide
6. Alertec
7. Armodafinil
8. Benzhydrylsulfinylacetamide
9. Crl 40476
10. Crl-40476
11. Modiodal
12. Nuvigil
13. Provigil
14. R Modafinil
15. R-modafinil
16. Sparlon
1. 68693-11-8
2. Provigil
3. Modiodal
4. 2-[(diphenylmethyl)sulfinyl]acetamide
5. Modafinilum [latin]
6. 2-(benzhydrylsulfinyl)acetamide
7. 2-benzhydrylsulfinylacetamide
8. Modafinilo [spanish]
9. Crl 40476
10. Modaphonil
11. Alertec
12. R Modafinil
13. Crl-40476
14. 2-((diphenylmethyl)sulfinyl)acetamide
15. Cep 1538
16. Modavigil
17. Modafinil Civ
18. C15h15no2s
19. Cep-1538
20. Nsc-751178
21. Nsc-759110
22. Chembl1373
23. R3uk8x3u3d
24. Acetamide, 2-((diphenylmethyl)sulfinyl)-
25. 112111-49-6
26. Chebi:77585
27. Thn102 Component Modafinil
28. Dep-1538
29. Thn-102 Component Modafinil
30. Crc-40476
31. 2-(diphenyl-methanesulfinyl)-acetamide
32. Ncgc00095176-03
33. Sparlon
34. Dsstox_cid_3329
35. Modafinil [usan:inn]
36. Dsstox_rid_76980
37. Dsstox_gsid_23329
38. (+-)-modafinil
39. Modafinilo
40. Modafinilum
41. Moderateafinil
42. Smr000058957
43. Cas-68693-11-8
44. (+/-)-modafinil
45. Sr-01000759419
46. 152jrg3t0u
47. Nh 02d
48. Nh-02d
49. Unii-r3uk8x3u3d
50. Attenace
51. Modalert
52. Modasomil
53. Crl 40983
54. Crl-40983
55. Dea No. 1680
56. Hsdb 7585
57. Modafinil [usan:usp:inn:ban]
58. Modafinil- Bio-x
59. 2-(diphenylmethyl)sulfinylacetamide
60. Provigil (tn)
61. Mfcd00868082
62. Modafinil [inn]
63. Modafinil [jan]
64. Modafinil [mi]
65. Modafinil [hsdb]
66. Modafinil [usan]
67. Spectrum2_001712
68. Modafinil [vandf]
69. Modafinil [mart.]
70. Modafinil [who-dd]
71. Modafinil (jan/usp/inn)
72. Unii-152jrg3t0u
73. Schembl34488
74. Bspbio_002270
75. Mls000759427
76. Mls001424049
77. Bidd:gt0714
78. Spectrum1505361
79. (-)benzhydrylsulphinylacetamide
80. Spbio_001724
81. Gtpl7555
82. Modafinil [orange Book]
83. Modafinil Civ [usp-rs]
84. Dtxsid0023329
85. Modafinil [ep Monograph]
86. Modafinil, >=98% (hplc)
87. Aft-801
88. Modafinil [usp Monograph]
89. Hms1922f12
90. Hms2051p17
91. Hms2093d15
92. Hms3264i03
93. Hms3393p17
94. Hms3713c12
95. Pharmakon1600-01504283
96. Pharmakon1600-01505361
97. 2-(diphenylmethane)sulfinylacetamide
98. 2-(diphenylmethyl)sulfinylethanamide
99. Bcp28422
100. Tox21_111470
101. Bdbm50156055
102. Ccg-39511
103. Cn 801
104. Cn-801
105. Grl 40476
106. Hy-15201a
107. Nsc751178
108. Nsc751850
109. Nsc758711
110. Nsc759110
111. 2-[di(phenyl)methylsulfinyl]acetamide
112. Modafinil 1.0 Mg/ml In Acetonitrile
113. Akos004118314
114. Tox21_111470_1
115. Ac-1382
116. Db00745
117. Nc00224
118. Nsc 751178
119. Nsc 759110
120. Ncgc00095176-01
121. Ncgc00095176-02
122. Ncgc00095176-04
123. Ncgc00095176-05
124. Ncgc00095176-06
125. Ncgc00095176-08
126. As-17039
127. Bm164601
128. Sbi-0206841.p001
129. Db-116344
130. Am20060710
131. D01832
132. Ab00639993_03
133. Ab00639993_04
134. 693m118
135. A836219
136. L001269
137. Q410441
138. Sr-01000759419-3
139. Sr-01000759419-4
140. Brd-a16332958-001-02-4
141. Brd-a16332958-001-04-0
142. F2173-0797
143. Modafinil, European Pharmacopoeia (ep) Reference Standard
144. Modafinil For System Suitability, European Pharmacopoeia (ep) Reference Standard
145. Modafinil Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
146. Alertec Pound>> Modavigil Pound>> Modiodal Pound>> Provigil Pound>> Modalert Pound>> Crl-40476
| Molecular Weight | 273.4 g/mol |
|---|---|
| Molecular Formula | C15H15NO2S |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 273.08234989 g/mol |
| Monoisotopic Mass | 273.08234989 g/mol |
| Topological Polar Surface Area | 79.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 302 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Modafinil |
| PubMed Health | Modafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | PROVIGIL (modafinil) is a wakefulness-promoting agent for oral administration. Modafinil is a racemic compound. The chemical name for modafinil is 2-[(diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the molecular weight is... |
| Active Ingredient | Modafinil |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 200mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Alembic; Apotex; Aurobindo Pharma; Sandoz; Hikma Pharms; Teva Pharms; Caraco; Carlsbad; Orchid Hlthcare; Barr |
| 2 of 4 | |
|---|---|
| Drug Name | Provigil |
| PubMed Health | Modafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | PROVIGIL (modafinil) is a wakefulness-promoting agent for oral administration. Modafinil is a racemic compound. The chemical name for modafinil is 2-[(diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the molecular weight is... |
| Active Ingredient | Modafinil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Cephalon |
| 3 of 4 | |
|---|---|
| Drug Name | Modafinil |
| PubMed Health | Modafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | PROVIGIL (modafinil) is a wakefulness-promoting agent for oral administration. Modafinil is a racemic compound. The chemical name for modafinil is 2-[(diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the molecular weight is... |
| Active Ingredient | Modafinil |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 200mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Alembic; Apotex; Aurobindo Pharma; Sandoz; Hikma Pharms; Teva Pharms; Caraco; Carlsbad; Orchid Hlthcare; Barr |
| 4 of 4 | |
|---|---|
| Drug Name | Provigil |
| PubMed Health | Modafinil (By mouth) |
| Drug Classes | CNS Stimulant |
| Drug Label | PROVIGIL (modafinil) is a wakefulness-promoting agent for oral administration. Modafinil is a racemic compound. The chemical name for modafinil is 2-[(diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the molecular weight is... |
| Active Ingredient | Modafinil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Cephalon |
Modafinil is indicated to improve wakefulness in patients with excessive daytime sleepiness associated with narcolepsy, obstructive sleep apnea/hypopnea (OSAHS), or shift work sleep disorder (SWSD). In OSAHS, modafinil is indicated as an adjunct to standard treatment. Continuing efficacy beyond 9 weeks has not been evaluated in placebo-controlled trials. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2019
Unlabeled uses: Treatment of fatigue associated with multiple sclerosis.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 1084
Modafinil is a wake-promoting agent shown to improve wakefulness in patients with excessive sleepiness (hypersomnolence) associated with shift work sleep disorder, obstructive sleep apnea, or narcolepsy. Safety and tolerability data from 6 randomized, double-blind, placebo-controlled studies were combined to evaluate modafinil across these different patient populations. One thousand five hundred twenty-nine outpatients received modafinil 200, 300, or 400 mg or placebo once daily for up to 12 weeks. Assessments included recording of adverse events and effects of modafinil on blood pressure/heart rate, electrocardiogram intervals, polysomnography, and clinical laboratory parameters. Two hundred seventy-three patients with shift work sleep disorder, 292 with obstructive sleep apnea, and 369 with narcolepsy received modafinil; 567 received placebo. Modafinil was well tolerated versus placebo, with headache (34% vs 23%, respectively), nausea (11% vs 3%), and infection (10% vs 12%) the most common adverse events. Adverse events were similar across all patient groups. Twenty-seven serious adverse events were reported (modafinil, n = 18; placebo, n = 9). In modafinil-treated patients, clinically significant increases in diastolic or systolic blood pressure were infrequent (n = 9 and n = 1, respectively, < 1% of patients). In the studies, 1 patient in the modafinil group and 1 in the placebo group had a clinically significant increase in heart rate. New clinically meaningful electrocardiogram abnormalities were rare with modafinil (n = 2) and placebo (n = 4). Clinically significant abnormalities in mean laboratory parameters were observed in fewer than 1% of modafinil-treated patients at final visit. Modafinil did not affect sleep architecture in any patient population according to polysomnography. Modafinil is well tolerated in the treatment of excessive sleepiness associated with disorders of sleep and wakefulness and does not affect cardiovascular or sleep parameters.
PMID:17993041 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2045706 Roth T et al; J Clin Sleep Med 3 (6): 595-602 (2007)
/EXPL THER/: ... a randomized, placebo-controlled crossover trial /was used/ ... to determine if modafinil can improve fatigue in patients with post-polio syndrome. Intervention with modafinil (400 mg/day) and placebo occurred over 6-week periods. Primary endpoint (fatigue) was assessed using the Fatigue Severity Scale as the main outcome measure. Other measures included the Visual Analog Scale for Fatigue and the Fatigue Impact Scale. Secondary endpoint (health-related quality of life) was assessed using the 36-Item Short-Form. Analysis of variance for repeated measures was applied to assess treatment, period, and carryover effects. Thirty-six patients were randomized, 33 of whom (mean age: 61 years) completed required interventions. Treatment with modafinil was safe and well-tolerated. After adjusting for periods and order effects, no difference was observed between treatments. Based on the utilized measures of outcome modafinil was not superior to placebo in alleviating fatigue or improving quality of life in the studied post-polio syndrome population.
PMID:17502549 Vasconcelos OM et al; Neurology 68 (20): 1680-6 (2007)
For more Therapeutic Uses (Complete) data for MODAFINIL (7 total), please visit the HSDB record page.
Serious rash, including Stevens-Johnson Syndrome requiring hospitalization and discontinuation of treatment has been reported in adults and children in association with the use of modafinil. ... Rare cases of serious or life-threatening rash, including Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis, and Drug Rash with Eosinophilia and Systemic Symptoms have been reported in adults and children in worldwide post-marketing experience. The reporting rate of Toxic Epidermal Necrolysis and Stevens-Johnson Syndrome associated with modafinil use, which is generally accepted to be an underestimate due to underreporting, exceeds the background incidence rate. Estimates of the background incidence rate for these serious skin reactions in the general population range between 1 to 2 cases per million-person years. There are no factors that are known to predict the risk of occurrence or the severity of rash associated with modafinil. Nearly all cases of serious rash associated with modafinil occurred within 1 to 5 weeks after treatment initiation. However, isolated cases have been reported after prolonged treatment (e.g., 3 months). Accordingly, duration of therapy cannot be relied upon as a means to predict the potential risk heralded by the first appearance of a rash. Although benign rashes also occur with modafinil, it is not possible to reliably predict which rashes will prove to be serious. Accordingly, modafinil should ordinarily be discontinued at the first sign of rash, unless the rash is clearly not drug-related. Discontinuation of treatment may not prevent a rash from becoming life-threatening or permanently disabling or disfiguring.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3467
Multi-organ hypersensitivity reactions, including at least one fatality in postmarketing experience, have occurred in close temporal association (median time to detection 13 days: range 4-33) to the initiation of modafinil. Although there have been a limited number of reports, multi-organ hypersensitivity reactions may result in hospitalization or be life-threatening. There are no factors that are known to predict the risk of occurrence or the severity of multi-organ hypersensitivity reactions associated with modafinil. Signs and symptoms of this disorder were diverse; however, patients typically, although not exclusively, presented with fever and rash associated with other organ system involvement. Other associated manifestations included myocarditis, hepatitis, liver function test abnormalities, hematological abnormalities (e.g., eosinophilia, leukopenia, thrombocytopenia), pruritis, and asthenia. Because multi-organ hypersensitivity is variable in its expression, other organ system symptoms and signs, not noted here, may occur.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3468
Psychiatric adverse experiences have been reported in patients treated with modafinil. Postmarketing adverse events associated with the use of modafinil have included mania, delusions, hallucinations, and suicidal ideation, some resulting in hospitalization. Many, but not all, patients had a prior psychiatric history. One healthy male volunteer developed ideas of reference, paranoid delusions, and auditory hallucinations in association with multiple daily 600 mg doses of modafinil and sleep deprivation. There was no evidence of psychosis 36 hours after drug discontinuation.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3468
Although modafinil has not been shown to cause functional impairment, the possibility that the drug, like any other drug affecting the CNS, may alter judgment, thinking, or motor skills should be considered. Patients should be cautioned about operating an automobile or other hazardous machinery until they are reasonably certain that modafinil does not adversely affect their ability to engage in such activities.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2494
For more Drug Warnings (Complete) data for MODAFINIL (20 total), please visit the HSDB record page.
To improve wakefulness in patients with excessive daytime sleepiness (EDS) associated with narcolepsy.
FDA Label
Modafinil is a stimulant drug marketed as a 'wakefulness promoting agent' and is one of the stimulants used in the treatment of narcolepsy. Narcolepsy is caused by dysfunction of a family of wakefulness-promoting and sleep-suppressing peptides, the orexins, whose neurons are activated by modafinil. The prexin neuron activation is associated with psychoactivation and euphoria. Modafinil is not indicated for complaints of lack of energy or fatigue; but it appears to be very helpful for some patients. Also, it has been used in the treatment of hypersomnia, a disorder in which patients lack the capacity for meaningful sleep and may require ten or more hours per day. Recent studies have have found that modafinil may help recovering cocaine addicts fight their addiction.
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Wakefulness-Promoting Agents
A specific category of drugs that prevent sleepiness by specifically targeting sleep-mechanisms in the brain. They are used to treat DISORDERS OF EXCESSIVE SOMNOLENCE such as NARCOLEPSY. Note that this drug category does not include broadly-acting central nervous system stimulants such as AMPHETAMINES. (See all compounds classified as Wakefulness-Promoting Agents.)
N06BA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06B - Psychostimulants, agents used for adhd and nootropics
N06BA - Centrally acting sympathomimetics
N06BA07 - Modafinil
Absorption
Rapid following oral administration.
Route of Elimination
The major route of elimination is metabolism (~90%), primarily by the liver, with subsequent renal elimination of the metabolites.
Volume of Distribution
0.9 L/kg
An open-label, single-center, single-dose, parallel-group study was performed in healthy young males and females as well as healthy elderly males to examine the influence of age and gender on the pharmacokinetics of modafinil following administration of a single 200 mg oral dose. Twelve subjects were enrolled in each of the following three groups: young males, young females, and elderly males. Each fasted (overnight) subject received 2 x 100 mg modafinil tablets. Blood and urine samples were collected at various times up to 72 hours postdose for the determination of plasma and urine levels of modafinil as well as the acid and sulfone metabolites. The plasma concentrations of the individual isomers, d- and l-modafinil, were also determined. Pharmacokinetic parameters were determined by noncompartmental methods. ... Modafinil was rapidly absorbed after oral dosing and slowly cleared (half life approximately 11-14 hr) from the body. Modafinil acid was the major urinary metabolite, which accounted for 35% to 60% of the dose. Results from this study indicated that there were age and gender effects on modafinil clearance processes. In this regard, the clearance rate of modafinil in males decreased with age while young females cleared modafinil at a faster rate than young males. Stereospecific pharmacokinetics of modafinil were also demonstrated. The d-modafinil was eliminated three times faster than the l-modafinil.
PMID:10073328 Wong YN et al; J Clin Pharmacol 39 (3): 281-8 (1999)
It is not known whether modafinil or its metabolites are distributed into milk.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2007., p. 2495
Modafinil is well distributed in body tissue with an apparent volume of distribution (approximately 0.9 L/kg) larger than the volume of total body water (0.6 L/kg). In human plasma, in vitro, modafinil is moderately bound to plasma protein (approximately 60%, mainly to albumin). At serum concentrations obtained at steady state after doses of 200 mg/day, modafinil exhibits no displacement of protein binding of warfarin, diazepam or propranolol. Even at much larger concentrations (1000uM; > 25 times the Cmax of 40uM at steady state at 400 mg/day), modafinil has no effect on warfarin binding. Modafinil acid at concentrations >500 uM decreases the extent of warfarin binding, but these concentrations are >35 times those achieved therapeutically.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3466
Absorption of Provigil tablets is rapid, with peak plasma concentrations occurring at 2-4 hours. The bioavailability of Provigil tablets is approximately equal to that of an aqueous suspension. The absolute oral bioavailability was not determined due to the aqueous insolubility (<1 mg/mL) of modafinil, which precluded intravenous administration. Food has no effect on overall Provigil bioavailability; however, its absorption (tmax) may be delayed by approximately one hour if taken with food.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3466
Modafinil is a racemic compound, whose enantiomers have different pharmacokinetics (e.g., the half-life of the l-isomer is approximately 3 times that of the d-isomer in humans). The enantiomers do not interconvert. At steady state, total exposure to the l-isomer is approximately 3 times that for the d-isomer. The trough concentration (Cmin ss) of circulating modafinil after once daily dosing consists of 90% of the l-isomer and 10% of the d-isomer.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 1085
Hepatic
The major route of elimination is metabolism (approximately 90%), primarily by the liver, with subsequent renal elimination of the metabolites. Urine alkalinization has no effect on the elimination of modafinil. Metabolism occurs through hydrolytic deamidation, S-oxidation, aromatic ring hydroxylation, and glucuronide conjugation. Less than 10% of an administered dose is excreted as the parent compound. In a clinical study using radiolabeled modafinil, a total of 81% of the administered radioactivity was recovered in 11 days post-dose, predominantly in the urine (80% vs. 1.0% in the feces). The largest fraction of the drug in urine was modafinil acid but at least six other metabolites were present in lower concentrations. Only two metabolites reach appreciable concentrations in plasma, i.e., modafinil acid and modafinil sulfone. In preclinical models, modafinil acid, modafinil sulfone, 2-((diphenylmethyl)sulfonyl)acetic acid and 4-hydroxy modafinil, were inactive or did not appear to mediate the arousal effects of modafinil.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3466
Based on in vitro data, modafinil is metabolized partially by the 3A isoform subfamily of hepatic cytochrome P450 (CYP3A4).
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3466
23-215 hours
Elimination: Effectively, about 15 hours after multiple dosing; the elimination half life of the levo-isomer is about three times that of the dextro-isomer.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007., p. 2020
... Modafinil was rapidly absorbed after oral dosing and slowly cleared (half life approximately 11-14 hr) from the body.
PMID:10073328 Wong YN et al; J Clin Pharmacol 39 (3): 281-8 (1999)
The exact mechanism of action is unclear, although in vitro studies have shown it to inhibit the reuptake of dopamine by binding to the dopamine reuptake pump, and lead to an increase in extracellular dopamine. Modafinil activates glutamatergic circuits while inhibiting GABA. Modafinil is thought to have less potential for abuse than other stimulants due to the absence of any significant euphoric or pleasurable effects. It is possible that modafinil acts by a synergistic combination of mechanisms including direct inhibition of dopamine reuptake, indirect inhibition of noradrenalin reuptake in the VLPO and orexin activation. Modafinil has partial alpha 1B-adrenergic agonist effects by directly stimulating the receptors.
The precise mechanism(s) through which modafinil promotes wakefulness is unknown. Modafinil has wake-promoting actions similar to sympathomimetic agents like amphetamine and methylphenidate, although the pharmacologic profile is not identical to that of sympathomimetic amines. Modafinil has weak to negligible interactions with receptors for norepinephrine, serotonin, dopamine, GABA, adenosine, histamine-3, melatonin, and benzodiazepines. Modafinil also does not inhibit the activities of MAO-B or phosphodiesterases II-V. Modafinil-induced wakefulness can be attenuated by the 1-adrenergic receptor antagonist prazosin; however, modafinil is inactive in other in vitro assay systems known to be responsive to -adrenergic agonists, such as the rat vas deferens preparation. Modafinil is not a direct- or indirect-acting dopamine receptor agonist. However, in vitro, modafinil binds to the dopamine transporter and inhibits dopamine reuptake. This activity has been associated in vivo with increased extracellular dopamine levels in some brain regions of animals. In genetically engineered mice lacking the dopamine transporter (DAT), modafinil lacked wake-promoting activity, suggesting that this activity was DAT-dependent. However, the wake-promoting effects of modafinil, unlike those of amphetamine, were not antagonized by the dopamine receptor antagonist haloperidol in rats. In addition, alpha-methyl-p-tyrosine, a dopamine synthesis inhibitor, blocks the action of amphetamine, but does not block locomotor activity induced by modafinil.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 3466
Modafinil is a well-tolerated medication for excessive sleepiness, attention-deficit disorder, cocaine dependence and as an adjunct to antidepressants with low propensity for abuse. ... The modafinil action on identified dopaminergic and GABAergic neurons in the ventral tegmental area (VTA) and substantia nigra (SN) of rat brain slices /was examined/. Modafinil (20 microM) inhibited the firing of dopaminergic, but not GABAergic neurons. This inhibition was maintained in the presence of tetrodotoxin and was accompanied by hyperpolarization. Sulpiride (10 microM), a D2-receptor antagonist, but not prazosine (20 microM, an alpha1-adrenoreceptor blocker) abolished the modafinil action. Inhibition of dopamine reuptake with a low dose of nomifensine (1 microM) reduced the firing of DA neurons in a sulpiride-dependent manner and blunted the effect of modafinil. On acutely isolated neurons, modafinil evoked D2-receptor-mediated outward currents in tyrosine-hydroxylase positive cells, identified by single-cell RT-PCR, which reversed polarity near the K(+) equilibrium potential and were unchanged in the presence of nomifensine. Thus modafinil directly inhibits DA neurons through D2 receptors.
PMID:17070873 Korotkova TM et al; Neuropharmacology 52 (2): 626-33 (2007)
Modafinil is a wakefulness-promoting drug that improves the alertness levels in narcolepsy; however, the molecular mechanism of action remains to be elucidated. We found that after a single icv injection of modafinil (10 ug/5 uL) the extracellular levels of dopamine (DA) and l-DOPA collected from the nucleus accumbens were increased and decreased, respectively. Separately, the icv administration of modafinil (10 microg/5 uL) to rats enhanced wakefulness (W) whereas diminished sleep during 4hr. Lastly, the alertness induced by modafinil was partially antagonized by the sleep-inducing endocannabinoid anandamide (ANA). We conclude that modafinil enhances the extracellular levels of DA, promotes W and its effects on sleep are partially blocked by ANA.
PMID:17098298 Murillo-Rodriguez E et al; Behav Brain Res 176 (2): 353-7 (2007)
Modafinil is an increasingly popular wake-promoting drug used for the treatment of narcolepsy, but its precise mechanism of action is unknown. To determine potential pathways via which modafinil acts, we administered a range of doses of modafinil to rats, recorded sleep/wake activity, and studied the pattern of neuronal activation using Fos immunohistochemistry. To contrast modafinil-induced wakefulness with spontaneous wakefulness, we administered modafinil at midnight, during the normal waking period of rats. To determine the influence of circadian phase or ambient light, we also injected modafinil at noon on a normal light/dark cycle or in constant darkness. We found that 75 mg/kg modafinil increased Fos immunoreactivity in the tuberomammillary nucleus (TMN) and in orexin (hypocretin) neurons of the perifornical area, two cell groups implicated in the regulation of wakefulness. This low dose of modafinil also increased the number of Fos-immunoreactive (Fos-IR) neurons in the lateral subdivision of the central nucleus of the amygdala. Higher doses increased the number of Fos-IR neurons in the striatum and cingulate cortex. In contrast to previous studies, modafinil did not produce statistically significant increases in Fos expression in either the suprachiasmatic nucleus or the anterior hypothalamic area. These observations suggest that modafinil may promote waking via activation of TMN and orexin neurons, two regions implicated in the promotion of normal wakefulness. Selective pharmacological activation of these hypothalamic regions may represent a novel approach to inducing wakefulness.
PMID:11069971 Scammell TE et al; J Neurosci 20 (22): 8620-8 (2000)
For more Mechanism of Action (Complete) data for MODAFINIL (6 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
77
PharmaCompass offers a list of Modafinil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Modafinil manufacturer or Modafinil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Modafinil manufacturer or Modafinil supplier.
PharmaCompass also assists you with knowing the Modafinil API Price utilized in the formulation of products. Modafinil API Price is not always fixed or binding as the Modafinil Price is obtained through a variety of data sources. The Modafinil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Provigil manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Provigil, including repackagers and relabelers. The FDA regulates Provigil manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Provigil API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Provigil manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Provigil supplier is an individual or a company that provides Provigil active pharmaceutical ingredient (API) or Provigil finished formulations upon request. The Provigil suppliers may include Provigil API manufacturers, exporters, distributors and traders.
click here to find a list of Provigil suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Provigil DMF (Drug Master File) is a document detailing the whole manufacturing process of Provigil active pharmaceutical ingredient (API) in detail. Different forms of Provigil DMFs exist exist since differing nations have different regulations, such as Provigil USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Provigil DMF submitted to regulatory agencies in the US is known as a USDMF. Provigil USDMF includes data on Provigil's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Provigil USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Provigil suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Provigil Drug Master File in Japan (Provigil JDMF) empowers Provigil API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Provigil JDMF during the approval evaluation for pharmaceutical products. At the time of Provigil JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Provigil suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Provigil Drug Master File in Korea (Provigil KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Provigil. The MFDS reviews the Provigil KDMF as part of the drug registration process and uses the information provided in the Provigil KDMF to evaluate the safety and efficacy of the drug.
After submitting a Provigil KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Provigil API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Provigil suppliers with KDMF on PharmaCompass.
A Provigil CEP of the European Pharmacopoeia monograph is often referred to as a Provigil Certificate of Suitability (COS). The purpose of a Provigil CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Provigil EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Provigil to their clients by showing that a Provigil CEP has been issued for it. The manufacturer submits a Provigil CEP (COS) as part of the market authorization procedure, and it takes on the role of a Provigil CEP holder for the record. Additionally, the data presented in the Provigil CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Provigil DMF.
A Provigil CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Provigil CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Provigil suppliers with CEP (COS) on PharmaCompass.
A Provigil written confirmation (Provigil WC) is an official document issued by a regulatory agency to a Provigil manufacturer, verifying that the manufacturing facility of a Provigil active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Provigil APIs or Provigil finished pharmaceutical products to another nation, regulatory agencies frequently require a Provigil WC (written confirmation) as part of the regulatory process.
click here to find a list of Provigil suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Provigil as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Provigil API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Provigil as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Provigil and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Provigil NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Provigil suppliers with NDC on PharmaCompass.
Provigil Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Provigil GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Provigil GMP manufacturer or Provigil GMP API supplier for your needs.
A Provigil CoA (Certificate of Analysis) is a formal document that attests to Provigil's compliance with Provigil specifications and serves as a tool for batch-level quality control.
Provigil CoA mostly includes findings from lab analyses of a specific batch. For each Provigil CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Provigil may be tested according to a variety of international standards, such as European Pharmacopoeia (Provigil EP), Provigil JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Provigil USP).
