
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
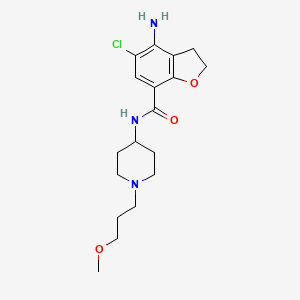

1. 4-amino-5-chloro-n-(1-(3-methoxypropyl)-4-piperidinyl)-2,3-dihydro-1-benzofuran-7-carboxamide
2. Motegrity
3. R 093877
4. R093877
5. Resolor
6. Resotran
7. Resotrans
1. 179474-81-8
2. Motegrity
3. R093877
4. 4-amino-5-chloro-2,3-dihydro-n-[1-(3-methoxypropyl)-4-piperidinyl]-7-benzofurancarboxamide
5. 4-amino-5-chloro-n-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboxamide
6. 4-amino-5-chloro-n-(1-(3-methoxypropyl)piperidin-4-yl)-2,3-dihydrobenzofuran-7-carboxamide
7. R-093877
8. 0a09iuw5tp
9. Chembl117287
10. 179474-81-8 (free Base)
11. Resotrans
12. Unii-0a09iuw5tp
13. R-93877
14. Prucalopride [usan:inn:ban]
15. Prucalopride [mi]
16. Prucalopride (usan/inn)
17. Prucalopride [inn]
18. Prucalopride [usan]
19. Dsstox_cid_31459
20. Dsstox_rid_97345
21. Dsstox_gsid_57670
22. Schembl16952
23. Gtpl243
24. Prucalopride [mart.]
25. Prucalopride [who-dd]
26. Prucalopride [ema Epar]
27. Dtxsid5057670
28. Chebi:135552
29. Bcpp000099
30. Hms3651f04
31. Hms3748m09
32. Hms3884p20
33. Bcp02177
34. Ex-a1333
35. Zinc1891034
36. Tox21_113885
37. Bdbm50122872
38. Mfcd09837787
39. S2875
40. Akos015951096
41. Am84628
42. Ccg-268257
43. Db06480
44. Pb31402
45. 4-amino-5-chloro-2,3-dihydro-n-(1-(3-methoxypropyl)-4-piperidyl)-7-benzofurancarboxamide
46. Ncgc00253867-01
47. Ncgc00253867-02
48. 4-amino-5-chloro-n-(1-(3-methoxypropyl)-4-piperidinyl)-2,3-dihydro-1-benzofuran-7-carboxamide
49. Ac-23945
50. As-19549
51. Hy-14151
52. Cas-179474-81-8
53. Ft-0674128
54. P2467
55. Sw219198-2
56. A25458
57. D09205
58. Q68484
59. 474p818
60. L000891
61. Sr-01000945275
62. Sr-01000945275-1
63. 4-amino-5+k445-chloro-2,3-dihydro-benzofuran-7-carboxylic Acid [1-(3-methoxy-propyl)-piperidin-4-yl]-amide
64. 4-amino-5-chloro-2,3-dihydro-benzofuran-7-carboxylic Acid [1-(3-methoxy-propyl)-piperidin-4-yl]-amide
65. 7-benzofurancarboxamide, 4-amino-5-chloro-2,3-dihydro-n-[1-(3-methoxypropyl)-4-piperidinyl]-
| Molecular Weight | 367.9 g/mol |
|---|---|
| Molecular Formula | C18H26ClN3O3 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 367.1662694 g/mol |
| Monoisotopic Mass | 367.1662694 g/mol |
| Topological Polar Surface Area | 76.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 445 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Prucalopride is indicated for the treatment of chronic idiopathic constipation (CIC) in adults. CIC is one of the most common chronic functional gastrointestinal disorders worldwide. The diagnosis of this agent is very hard and it can be confirmed if the patient experience at least two of the following: -Straining during more than 25% of the bowel movements. -Lumpy or hard stools in 25% of the bowel movements. -Sensation of incomplete evacuation in more than 25% of all bowel movements. -Sensation of anorectal blockage or obstruction in more than 25% of the bowel movements. -Manual maneuvers required in more than 25% of the bowel movements. -Fewer than 3 bowel movements per week.
FDA Label
Resolor is indicated for symptomatic treatment of chronic constipation in adults in whom laxatives fail to provide adequate relief.
In animal studies, prucalopride induced a dose-dependent stimulation of contractile activity in the proximal colon and inhibition of the contractility in the distal colon. As well it has been shown that prucalopride stimulates and amplifies giant migratory contraction which is the high-amplitude type of contraction that initiates the urge to defecate. Thus, prucalopride not only accelerates the colonic transit but also accelerates gastric emptying and small bowel transit. In supratherapeutic concentrations, prucalopride can be observed to interact with hERG potassium channels and L-type calcium channels. In clinical trials, prucalopride showed to significantly increase the spontaneous bowel movements with a standardized mean difference of about 0.5 after the use of 1 mg when compared with the placebo group. In this studies as well, it was observed a numerical improvement in mean colonic transit time and a significant increase in spontaneous complete bowel movement without marked changes in the anorectal function. In phase III clinical trials, 86% of the tested individuals opted to continue with the open-label study and based on Patients Assessments, 67% of the patients increase more than one point improvement in their satisfaction. In the final set of clinical trials for approval, there was a significant increase in the number of patients that reached over 3 complete spontaneous bowel movements per week when compared with the placebo.
Laxatives
Agents that produce a soft formed stool, and relax and loosen the bowels, typically used over a protracted period, to relieve CONSTIPATION. (See all compounds classified as Laxatives.)
Serotonin 5-HT4 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT4 RECEPTORS. (See all compounds classified as Serotonin 5-HT4 Receptor Agonists.)
A06AX05
A06AX05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AX - Other drugs for constipation
A06AX05 - Prucalopride
Absorption
Prucalopride is well absorbed and it reaches maximum plasma concentration of 3.79ng/ml with a tmax of 2.77 hours after initial administration. It presents an AUC of 96.5 mn.h/ml. The bioavailability of prucalopride is of over 90% and this bioavailability does not get influenced by the ingestion of food.
Route of Elimination
After maximum plasma concentration, prucalopride concentration decline in a biphasic manner. Prucalopride is mainly excreted by the urine, representing 84% of the administered dose while only 13% of the dose is recovered in feces.
Volume of Distribution
The mean volume of distribution of prucalopride is registered to be 623 L.
Clearance
Prucalopride renal clearance is reported to be of 17 L/h which actually exceeds the glomerular filtration rate of the kidney.
Prucalopride is not extensively metabolized in the body and does not interact with the enzymes of the family of the cytochrome P450 enzymes nor the P glycoprotein. The metabolism of prucalopride only represents 6% of the administered dose and the remaining 94% is found as the unchanged drug. From studies, it was reported the recovery of 8 metabolites being the major metabolite R107504 which is formed after the O-demethylation and oxidation of the resulting alcohol to a carboxylic acid.
The reported half-life of prucalopride is of around 18-20 hours.
Prucalopride acts as a selective stimulator of the 5-HT4 receptors while having no interaction with hERG channel or 5-HT1 receptors which reduces significantly the cardiovascular risk found in other similar drugs. 5-HT4 receptors can be found throughout the gastrointestinal tract primarily in smooth muscle cells, enterochromaffin cells, and myenteric plexus. Its activation produces the release of acetylcholine which is the major excitatory neurotransmitter in the GI tract. Hence, prucalopride stimulates motility by interacting specifically with 5-HT4 receptors in the GI tract which causes a release of acetylcholine and further contraction of the muscle layer of the colon and relaxation of the circular muscle layer leading to the propulsion of luminal content.
Global Sales Information

ABOUT THIS PAGE
27
PharmaCompass offers a list of Prucalopride (USAN/INN) API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Prucalopride (USAN/INN) manufacturer or Prucalopride (USAN/INN) supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Prucalopride (USAN/INN) manufacturer or Prucalopride (USAN/INN) supplier.
PharmaCompass also assists you with knowing the Prucalopride (USAN/INN) API Price utilized in the formulation of products. Prucalopride (USAN/INN) API Price is not always fixed or binding as the Prucalopride (USAN/INN) Price is obtained through a variety of data sources. The Prucalopride (USAN/INN) Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Prucalopride (USAN/INN) manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Prucalopride (USAN/INN), including repackagers and relabelers. The FDA regulates Prucalopride (USAN/INN) manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Prucalopride (USAN/INN) API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Prucalopride (USAN/INN) supplier is an individual or a company that provides Prucalopride (USAN/INN) active pharmaceutical ingredient (API) or Prucalopride (USAN/INN) finished formulations upon request. The Prucalopride (USAN/INN) suppliers may include Prucalopride (USAN/INN) API manufacturers, exporters, distributors and traders.
Prucalopride (USAN/INN) Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Prucalopride (USAN/INN) GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Prucalopride (USAN/INN) GMP manufacturer or Prucalopride (USAN/INN) GMP API supplier for your needs.
A Prucalopride (USAN/INN) CoA (Certificate of Analysis) is a formal document that attests to Prucalopride (USAN/INN)'s compliance with Prucalopride (USAN/INN) specifications and serves as a tool for batch-level quality control.
Prucalopride (USAN/INN) CoA mostly includes findings from lab analyses of a specific batch. For each Prucalopride (USAN/INN) CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Prucalopride (USAN/INN) may be tested according to a variety of international standards, such as European Pharmacopoeia (Prucalopride (USAN/INN) EP), Prucalopride (USAN/INN) JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Prucalopride (USAN/INN) USP).
