
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
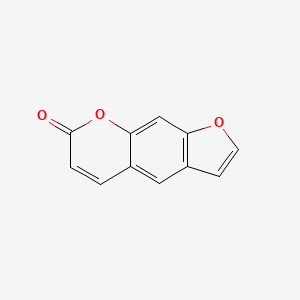

1. Ficusin
2. Psoralene
1. 66-97-7
2. Ficusin
3. 7h-furo[3,2-g]chromen-7-one
4. Furocoumarin
5. Psoralene
6. 7h-furo[3,2-g][1]benzopyran-7-one
7. Furo[3,2-g]chromen-7-one
8. Psorline-p
9. 6,7-furanocoumarin
10. Furo[3,2-g]coumarin
11. Furo(2',3',7,6)coumarin
12. Furo(3,2-g)-coumarin
13. Furo(4',5',6,7)coumarin
14. Nsc 404562
15. 7h-furo[3,2-g]benzopyran-7-one
16. Furo[2',3':7,6]coumarin
17. Furo[4',5':6,7]coumarin
18. 7h-furo(3,2-g)(1)benzopyran-7-one
19. Furo[2'.3':7.6]coumarin
20. Ktz7zcn2ex
21. 6-hydroxy-5-benzofuranacrylic Acid Beta-lactone
22. Nsc-404562
23. 5-benzofuranacrylic Acid, 6-hydroxy-, Delta-lactone
24. Chembl164660
25. Chebi:27616
26. 2h-furo[3,2-g]chromen-2-one
27. 6-hydroxy-5-benzofuranacrylic Acid Delta-lactone
28. Tnp00293
29. 6-hydroxy-5-benzofuranacrylic Acid Gamma-lactone
30. 3-(6-hydroxy-5-benzofuranyl)-2-propenoic Acid Delta-lactone
31. Ccris 4343
32. Hsdb 3528
33. Unii-ktz7zcn2ex
34. Einecs 200-639-7
35. Brn 0152784
36. Manaderm
37. Furano[3,2-g]chromen-2-one
38. Psoralene (dcf)
39. Manaderm (tn)
40. Psoralen,(s)
41. Mfcd00010520
42. Psoralen, 97%
43. Furo[4',7]coumarin
44. Phytoalexin-cmpd
45. Psoralen [hsdb]
46. Psoralen [mi]
47. 2-propenoic Acid, 3-(6-hydroxy-5-benzofuranyl)-, Delta-lactone
48. Psoralen [usp-rs]
49. Psoralen [who-dd]
50. Oprea1_841692
51. Schembl17835
52. Psoralen, Analytical Standard
53. 5-19-04-00445 (beilstein Handbook Reference)
54. Mls001304059
55. Megxp0_001172
56. Psoralen, >=99% (hplc)
57. Acon1_001579
58. Pyrano[5,6-f]benzofuran-7-one
59. Dtxsid00216205
60. 7-furo[3,2-g][1]benzopyranone
61. Hms2267l05
62. Zinc120283
63. Bcp04177
64. Hy-n0053
65. 7h-furo[3,2-g]chromen-7-one #
66. Bdbm50331544
67. Nsc404562
68. S4737
69. Stl564822
70. Akos004110987
71. Ac-7968
72. Ccg-266472
73. Cs-3756
74. Ncgc00017351-01
75. Ncgc00017351-02
76. Ncgc00017351-03
77. Ncgc00017351-07
78. Ncgc00142529-01
79. As-59034
80. Smr000112587
81. Ft-0603268
82. N1332
83. P2077
84. 7h-furo[3,2-g][1]benzopyran-7-one, 9ci
85. C09305
86. D08450
87. P-7850
88. 010p520
89. A835599
90. Q417788
91. Brd-k47264279-001-01-4
92. 5-benzofuranacrylic Acid, 6-hydroxy-, .delta.-lactone
93. 6-hydroxy-5-benzofuranacrylic Acid .delta.-lactone
94. 2-propenoic Acid, 3-(6-hydroxy-5-benzofuranyl)-, .delta.-lactone
| Molecular Weight | 186.16 g/mol |
|---|---|
| Molecular Formula | C11H6O3 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 186.031694049 g/mol |
| Monoisotopic Mass | 186.031694049 g/mol |
| Topological Polar Surface Area | 39.4 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 284 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Osteoporosis is a systemic skeletal disease, which is characterized by a systemic destruction of bone mass and microarchitecture. With life standard improved, the treatment of osteoporosis attracted more attention. The aim of this study is to verify the osteoprotective effect of psoralen and isopsoralen in females and males. Female and male mice were divided into 7 groups in this study: control group (sham-operation), model group (by ovariectomy or orchidectomy), positive control group (females given estradiol valerate; males given alendronate sodium), psoralen groups (10 mg/kg and 20 mg/kg), and isopsoralen groups (10 mg/kg and 20 mg/kg). After administration of psoralen and isopsoralen for 8 weeks, osteoporosis was ameliorated with increasing bone strength and improving trabecular bone microstructure as indicated by CT scan and pathology. Serum alkaline phosphatase (ALP), tartrate resistant acid phosphatase (TRACP), osteocalcin (OC), and C-terminal cross-linking telopeptides of type I collagen (CTX-1) were examined. Decreased TRACP and increased ALP/TRACP suggested restoring from bone destruction. These results suggest that psoralen and isopsoralen may be used as good natural compounds for the treatment of osteoporosis in males, as well as females.
PMID:27239473 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4867056 Yuan X et al; Biomed Res Int 2016: 6869452 (2016)
/EXPL THER/ This work investigates X-PACT (X-ray Psoralen Activated Cancer Therapy): a new approach for the treatment of solid cancer. X-PACT utilizes psoralen, a potent anti-cancer therapeutic with current application to proliferative disease and extracorporeal photopheresis (ECP) of cutaneous T Cell Lymphoma. An immunogenic role for light-activated psoralen has been reported, contributing to long-term clinical responses. Psoralen therapies have to-date been limited to superficial or extracorporeal scenarios due to the requirement for psoralen activation by UVA light, which has limited penetration in tissue. X-PACT solves this challenge by activating psoralen with UV light emitted from novel non-tethered phosphors (co-incubated with psoralen) that absorb x-rays and re-radiate (phosphoresce) at UV wavelengths. The efficacy of X-PACT was evaluated in both in-vitro and in-vivo settings. In-vitro studies utilized breast (4T1), glioma (CT2A) and sarcoma (KP-B) cell lines. Cells were exposed to X-PACT treatments where the concentrations of drug (psoralen and phosphor) and radiation parameters (energy, dose, and dose rate) were varied. Efficacy was evaluated primarily using flow cell cytometry in combination with complimentary assays, and the in-vivo mouse study. In an in-vitro study, we show that X-PACT induces significant tumor cell apoptosis and cytotoxicity, unlike psoralen or phosphor alone (p<0.0001). We also show that apoptosis increases as doses of phosphor, psoralen, or radiation increase. Finally, in an in-vivo pilot study of BALBc mice with syngeneic 4T1 tumors, we show that the rate of tumor growth is slower with X-PACT than with saline or AMT + X-ray (p<0.0001). Overall these studies demonstrate a potential therapeutic effect for X-PACT, and provide a foundation and rationale for future studies. In summary, X-PACT represents a novel treatment approach in which well-tolerated low doses of x-ray radiation are delivered to a specific tumor site to generate UVA light which in-turn unleashes both short- and potentially long-term antitumor activity of photo-active therapeutics like psoralen.
PMID:27583569 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5008763 Oldham M et al; PLoS One 11 (9): e0162078 (2016)
/EXPL THER/ Mycosis fungoides with large-cell transformation is historically associated with a poor prognosis. Pediatric cases of mycosis fungoides with large-cell transformation are rare, with only three other cases reported in the literature. We present the first case of a child with almost complete remission of his mycosis fungoides with large-cell transformation shortly after administration of psoralen plus ultraviolet A, interferon-alfa, and localized radiation.
PMID:29159918 Chan WH et al; Pediatr Dermatol 35 (1): e13-e16 (2018)
/EXPL THER/ BACKGROUND: Isotretinoin has been used in combination with oral psoralen+UVA (PUVA) and narrowband UVB (NBUVB) for treating psoriasis, especially in women of child-bearing age. The efficacy of oral psoralen+sun exposure (PUVAsol) is comparable to that of PUVA. This study was planned to compare the efficacy of oral PUVAsol with that of the combination of oral isotretinoin and PUVAsol in patients with chronic plaque psoriasis. METHODS: Forty patients with psoriasis vulgaris were randomized to two groups. Group A (control group) received PUVAsol only. Group B (intervention group) received PUVAsol+isotretinoin (0.5 mg/kg/day). Psoriasis Area Severity Index (PASI) score was recorded at baseline and weeks 4, 8 and 12. Dermatology Life Quality Index was assessed at baseline and 12 weeks. The end point of the study was PASI 75 or 12 weeks, whichever came earlier. RESULTS: Thirty-five patients completed the study. There were statistically significant differences between the two study groups for the number of patients achieving the endpoint of PASI 75, PASI scores at the end of 12 weeks, mean duration to achieve PASI 75, number of PUVAsol sessions needed to achieve PASI75 and mean cumulative dosage of 8-methoxypsoralen needed to achieve PASI 75. CONCLUSION: The combination of isotretinoin with PUVAsol is more effective compared with PUVAsol alone for treating chronic plaque psoriasis.
PMID:24828298 Gahalaut P et al; Photodermatol Photoimmunol Photomed 30 (6): 294-30 (2014)
For more Therapeutic Uses (Complete) data for Psoralen (14 total), please visit the HSDB record page.
This study was aimed to alert the hazard of accidental adverse reactions of photochemotherapy (Psoralen-UVA or PUVA) that has been used in the treatment for some skin diseases and commercially for cosmetic tanning. Aside from the predictable side effects of PUVA such as erythema and itching, the accidental adverse reactions such as extensive burns could occasionally occur. Our observations indicated that six cases resulted from mistakes of medical personnel, and six other cases resulted from unsupervised mistakes of patients. The conditions that needed photochemotherapy were seven cases of vitiligo, three cases of psoriasis and two cases of tanning. The accidental overdose of UV radiation was about 3-10 times the empirically normal dose. Five of our patients were supposed to undergo topical PUVA, but they were irradiated at the dose of oral PUVA. One patient applied 8-methoxypsoralen (8-MOP) cream together with taking 5-methoxypsoralen (5-MOP) tablets for oral PUVA. Three other patients enjoyed sunbathing 1-3h shortly after finishing PUVA. A young couple chose 5-MOP to enhance tanning and sunbathed about 1h later. When another patient resumed PUVA in a 6-month cessation, he was exposed at a previous dose instead of a starting dose. Erythema and blisters of second degree burns developed in all our cases, 36-72h after PUVA, with 5-25% of body surface involved. Among the 12 patients, 3 were admitted and 9 were treated on an outpatient basis. All patients recovered in 1-3 weeks with no skin graft or no significant sequelae except post-inflammatory hyperpigmentation.
PMID:17218059 Herr H et al; Burns 33 (3): 372-5 (2007)
POTENTIAL ADVERSE EFFECTS ON FETUS: Animal studies not performed. Unknown effect on humans. Should be given only if clearly needed. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: None known whether excreted. Should be avoided. FDA Category: C (C = Studies in laboratory animals have revealed adverse effects on the fetus (teratogenic, embryocidal, etc.) but there are no controlled studies in pregnant women. The benefits from use of the drug in pregnant women may be acceptable despite its potential risks, or there are no laboratory animal studies or adequate studies in pregnant women.) /Psoralens/ /from table II/
Stockton, D.L. and A.S. Paller. J Am Acad Dermatol 23 (1): 87-103 (1990)
Psoralen plus ultraviolet A irradiation (PUVA therapy) is commonly used for the management of vitiligo in which perifollicular repigmentation is the usual response pattern. However, excessive PUVA therapy may be associated with adverse effects. We report a case of generalized vitiligo that has been extensively treated with topical and systemic PUVA therapy for several years with the development of extensive and widespread stellate and irregularly shaped black and brown macules (lentigines). Interestingly, the lentigines were observed not only in the normally pigmented skin but also within the depigmented lesions that were lacking the perifollicular response pattern. The lesions developed in the exposed and unexposed skin areas. No evidence of skin malignancy was observed clinically and no melanocyte atypia was detected histopathologically. Cryotherapy may be used in the management of the lentigines; however, because of the extent of lesions this was impractical in our case.
PMID:15245535 Abdel Naser MB et al; Clin Exp Dermatol 29 (4): 380-2 (2004)
BACKGROUND: Changes in the appearance of the skin including actinic degeneration and pigmentary changes have been noted in patients treated with psoralen and UVA (PUVA). OBJECTIVE: Our purpose was to quantify risk factors for increased extent and progression of actinic degeneration and pigmentary changes in the skin of patients treated with PUVA. METHODS: On the basis of standardized dermatologic examination conducted in 1977 and 1998 of patients enrolled in the PUVA Follow Up Study, we assessed the prevalence of and changes in the extent of actinic degeneration and pigmentary abnormalities on the hands and buttocks. RESULTS: From 1977 to 1998, the prevalence of moderate or severe actinic degeneration increased from 15.6% to 60.5% on the hands and from 2.2% to 21.3% on the buttocks. During this same period, the prevalence of pigmentary changes of this degree increased from 15.6% to 58.6% on the hands and 12.6% to 24.7% on the buttocks. Extent of exposure to PUVA was the strongest predictor of an increased extent of clinical actinic degeneration or pigmentary change. CONCLUSION: Long-term exposure to PUVA is associated with persistent increases in actinic degeneration and pigmentary abnormalities of the skin on both usually sun-exposed and sun-protected sites.
PMID:12522372 Stern RS; J Am Acad Dermatol 48 (1): 61-7 (2003)
For more Drug Warnings (Complete) data for Psoralen (8 total), please visit the HSDB record page.
Anthelmintics
Agents that kill parasitic worms. They are used therapeutically in the treatment of HELMINTHIASIS in man and animal. (See all compounds classified as Anthelmintics.)
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
Cross-Linking Reagents
Reagents with two reactive groups, usually at opposite ends of the molecule, that are capable of reacting with and thereby forming bridges between side chains of amino acids in proteins; the locations of naturally reactive areas within proteins can thereby be identified; may also be used for other macromolecules, like glycoproteins, nucleic acids, or other. (See all compounds classified as Cross-Linking Reagents.)
OBJECTIVE: To investigate the nasal absorption regularities of psoralen and isopsoralen of different concentrations. METHOD: Building an experimental model of rat in situ nasal recirculation and determining the contents of psoralen and isopsoralen by HPLC. RESULT: The nasal absorption of psoralen and isopsoralen fitted in with zero order kinetics, getting saturated with the increase of concentration. CONCLUSION: A suitable concentration is necessary for the preparation of nasal remedies psoralen and isopsoralen.
PMID:12212091 Li A, Wang H; Zhongguo Zhong Yao Za Zhi 24 (11): 689-91, 704 (1999)
Psoralen has known human metabolites that include 5,7,11-Trioxatetracyclo[8.4.0.03,8.04,6]tetradeca-1,3(8),9,13-tetraen-12-one.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Market Place

ABOUT THIS PAGE
44
PharmaCompass offers a list of Psoralen API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Psoralen manufacturer or Psoralen supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Psoralen manufacturer or Psoralen supplier.
PharmaCompass also assists you with knowing the Psoralen API Price utilized in the formulation of products. Psoralen API Price is not always fixed or binding as the Psoralen Price is obtained through a variety of data sources. The Psoralen Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Psoralen manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Psoralen, including repackagers and relabelers. The FDA regulates Psoralen manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Psoralen API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Psoralen supplier is an individual or a company that provides Psoralen active pharmaceutical ingredient (API) or Psoralen finished formulations upon request. The Psoralen suppliers may include Psoralen API manufacturers, exporters, distributors and traders.
Psoralen Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Psoralen GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Psoralen GMP manufacturer or Psoralen GMP API supplier for your needs.
A Psoralen CoA (Certificate of Analysis) is a formal document that attests to Psoralen's compliance with Psoralen specifications and serves as a tool for batch-level quality control.
Psoralen CoA mostly includes findings from lab analyses of a specific batch. For each Psoralen CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Psoralen may be tested according to a variety of international standards, such as European Pharmacopoeia (Psoralen EP), Psoralen JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Psoralen USP).

