
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
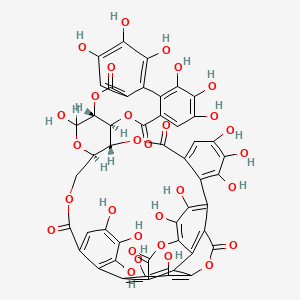

1. Punicalagin
1. Punicalagin
2. 65995-63-3
3. Punicalagin, Technical Grade
4. Mfcd09838017
5. A Pomegranate Ellagitannin
6. Punicalagin, Analytical Standard
7. Chebi:167695
8. Glxc-14665
9. Akos037514808
10. Punicalagin, >=98% (hplc), From Pomegranate
11. 995p633
12. Q-100754
13. (63r,64r,64as,616ar)-14,15,16,22,23,27,28,34,35,36,61,68,69,610,611,612,613-heptadecahydroxy-25,210,63,64,64a,66,615,616a-octahydro-61h-5,8-dioxa-6(4,3)-dibenzo[f,h]pyrano[3,4-b][1,4]dioxecina-2(1,6)-chromeno[5,4,3-cde]chromena-1,3(1,2)-dibenzenacyclononaphane-25,210,66,615,4,9-hexaone
14. 6,7,8,11,12,23,24,27,28,29,37,43,44,45,48,49,50-heptadecahydroxy-2,14,21,33,36,39,54-heptaoxaundecacyclo[33.20.0.04,9.010,19.013,18.016,25.017,22.026,31.038,55.041,46.047,52]pentapentaconta-4,6,8,10,12,16,18,22,24,26,28,30,41,43,45,47,49,51-octadecaene-3,15,20,32,40,53-hexone
| Molecular Weight | 1084.7 g/mol |
|---|---|
| Molecular Formula | C48H28O30 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 17 |
| Hydrogen Bond Acceptor Count | 30 |
| Rotatable Bond Count | 0 |
| Exact Mass | 1084.06653947 g/mol |
| Monoisotopic Mass | 1084.06653947 g/mol |
| Topological Polar Surface Area | 511 Ų |
| Heavy Atom Count | 78 |
| Formal Charge | 0 |
| Complexity | 2380 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Hydrolyzable Tannins
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
EXPL THER Human enterovirus 71 is one of the major causative agents of hand, foot and mouth disease in children and has caused mortalities in large-scale outbreaks in the Asia-Pacific region in recent years. No vaccine or antiviral therapy is available currently in the clinic. ...this work... investigated the antiviral effect of punicalagin on enterovirus 71 both in vitro and in vivo. The results showed that punicalagin reduced the viral cytopathic effect on rhabdomyosarcoma cells with an IC50 value of 15 ug/mL. Moreover, punicalagin treatment of mice challenged with a lethal dose of enterovirus 71 resulted in a reduction of mortality and relieved clinical symptoms by inhibiting viral replication. /This/ work suggested that punicalagin have the potential for further development as antiviral agents against enterovirus 71.
Yang Y et al; Phytomedicine Volume 20 (1): 67-70 (2012)
EXPL THER Since T cell activation is central to the development of autoimmune diseases, /the authors/ screened a natural product library comprising 1400 samples of medicinal herbal extracts, to identify compounds that suppress T cell activity. Punicalagin (PCG) isolated from the fruit of Punica granatum was identified as a potent immune suppressant, based on its inhibitory action on the activation of the nuclear factor of activated T cells (NFAT). PCG downregulated the mRNA and soluble protein expression of interleukin-2 from anti-CD3/anti-CD28-stimulated murine splenic CD4+ T cells and suppressed mixed leukocytes reaction (MLR) without exhibiting cytotoxicity to the cells. In vivo, the PCG treatment inhibited phorbol 12-myristate 13-acetate (PMA)-induced chronic ear edema in mice and decreased CD3+ T cell infiltration of the inflamed tissue. These results suggest that PCG could be a potential candidate for the therapeutics of various immune pathologies.
Lee S-I et al; Biochemical and Biophysical Research Communications 371 (4): 799-803 (2008)
... The present study evaluated the possible toxic effect of punicalagin in Sprague-Dawley rats upon repeated oral administration of a 6% punicalagin-containing diet for 37 days. Punicalagin and related metabolites were identified by HPLC-DAD-MS-MS in plasma, liver, and kidney. Five punicalagin-related metabolites were detected in liver and kidney, that is, two ellagic acid derivatives, gallagic acid, 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one glucuronide, and 3,8,10-trihydroxy-6H-dibenzo[b,d]pyran-6-one.
PMID:12744688 Cerda B et al; J Agric Food Chem. 51 (11): 3493-501 (2003)
Pomegranate, a fruit native to the Middle East, has gained widespread popularity as a functional food and nutraceutical source. The health effects of the whole fruit, as well as its juices and extracts, have been studied in relation to a variety of chronic diseases. Promising results against cardiovascular disease, diabetes, and prostate cancer have been reported from human clinical trials. The in vitro antioxidant activity of pomegranate has been attributed to its high polyphenolic content, specifically punicalagins, punicalins, gallagic acid, and ellagic acid. These compounds are metabolized during digestion to ellagic acid and urolithins, suggesting that the bioactive compounds that provide in vivo antioxidant activity may not be the same as those present in the whole food...
PMID:22129380 Johanningsmeier SD, Harris GK; Annu Rev Food Sci Technol. 2: 181-201 (2011)
Pomegranates have been shown to contain 124 different phytochemicals, and some of them act in concert to exert antioxidant and anti-inflammatory effects on cancer cells. Ellagitannins are bioactive polyphenols present in pomegranate. Pomegranate juice obtained by squeezing the whole fruit has the highest concentration of ellagitannins than any commonly consumed juice and contains the unique ellagitannin, punicalagin. Punicalagin is the known largest molecular weight polyphenol. Pomegranate ellagitannins are not absorbed intact into the blood stream but are hydrolyzed to ellagic acid over several hours in the intestine. Ellagitannins are also metabolized into urolithins by gut flora, which are conjugated in the liver and excreted in the urine. These urolithins are also bioactive and inhibit prostate cancer cell growth...
Heber D; in Herbal Medicine: Biomolecular and Clinical Aspects; Benzie IFF, Wachtel-Galor S, eds (2011)
Intraperitoneal and oral administration of synthesized urolithin A led to uptake of urolithin A and its conjugates in prostate tissue, and levels were higher in prostate, colon, and intestinal tissues relative to other organs. It is unclear why pomegranate ellagitannins metabolites localize at higher levels in the prostate, colon, and intestinal tissues relative to the other organs studied. Importantly, the predilection of bioactive pomegranate ellagitannins metabolites to localize in prostate tissue, combined with clinical data demonstrating the anticancer effects of pomegranate juice, suggests the potential for pomegranate products to play a role in prostate cancer chemoprevention. Whether uro-lithins in human prostate tissue can be used as a biomarker following the long-term administration of pomegranate juice or pomegranate extract remains to be determined. /pomegranate extract/
Heber D; in Herbal Medicine: Biomolecular and Clinical Aspects; Benzie IFF, Wachtel-Galor S, eds (2011)
... The present study evaluated the possible toxic effect of punicalagin in Sprague-Dawley rats upon repeated oral administration of a 6% punicalagin-containing diet for 37 days. Punicalagin and related metabolites were identified by HPLC-DAD-MS-MS in plasma, liver, and kidney. Five punicalagin-related metabolites were detected in liver and kidney, that is, two ellagic acid derivatives, gallagic acid, 3,8-dihydroxy-6H-dibenzo[b,d]pyran-6-one glucuronide, and 3,8,10-trihydroxy-6H-dibenzo[b,d]pyran-6-one.
PMID:12744688 Cerda B et al; J Agric Food Chem. 51 (11): 3493-501 (2003)
Several fruit juices have been reported to cause food-drug interactions, mainly affecting cytochrome P450 activity; however, little is known about the effects of fruit juices on conjugation reactions. Among several fruit juices tested (apple, peach, orange, pineapple, grapefruit, and pomegranate), pomegranate juice potently inhibited the sulfoconjugation of 1-naphthol in Caco-2 cells. This inhibition was both dose- and culture time-dependent, with a 50% inhibitory concentration (IC(50)) value calculated at 2.7% (vol/vol). In contrast, no obvious inhibition of glucuronidation of 1-naphthol in Caco-2 cells was observed by any of the juices examined. Punicalagin, the most abundant antioxidant polyphenol in pomegranate juice, was also found to strongly inhibit sulfoconjugation in Caco-2 cells with an IC(50) of 45 uM, which is consistent with that of pomegranate juice. These data suggest that punicalagin is mainly responsible for the inhibition of sulfoconjugation by pomegranate juice. /The authors/ additionally demonstrated that pomegranate juice and punicalagin both inhibit phenol sulfotransferase activity in Caco-2 cells in vitro, at concentrations that are almost equivalent to those used in the Caco-2 cells. Pomegranate juice, however, shows no effects on the expression of the sulfotransferase SULT1A family of genes (SULT1A1 and SULT1A3) in Caco-2 cells. These results indicate that the inhibition of sulfotransferase activity by punicalagin in Caco-2 cells is responsible for the reductions seen in 1-naphthyl sulfate accumulation. /The/ data also suggest that constituents of pomegranate juice, most probably punicalagin, impair the enteric functions of sulfoconjugation and that this might have effects upon the bioavailability of drugs and other compounds present in food and in the environment. These effects might be related to the anticarcinogenic properties of pomegranate juice.
PMID:19053852 Saruwatari A et al; J Med Food. 11 (4): 623-8 (2008)
Polyphenol-rich dietary foodstuffs have attracted attention due to their cancer chemopreventive and chemotherapeutic properties. Ellagitannins (ETs) belong to the so-called hydrolysable tannins found in strawberries, raspberries, walnuts, pomegranate, oak-aged red wine, etc. Both ETs and their hydrolysis product, ellagic acid (EA), have been reported to induce apoptosis in tumour cells. Ellagitannins are not absorbed in vivo but reach the colon and release EA that is metabolised by the human microflora. Our aim was to investigate the effect of a dietary ET [pomegranate punicalagin (PUNI)] and EA on human colon cancer Caco-2 and colon normal CCD-112CoN cells. Both PUNI and EA provoked the same effects on Caco-2 cells: down-regulation of cyclins A and B1 and upregulation of cyclin E, cell-cycle arrest in S phase, induction of apoptosis via intrinsic pathway (FAS-independent, caspase 8-independent) through bcl-XL down-regulation with mitochondrial release of cytochrome c into the cytosol, activation of initiator caspase 9 and effector caspase 3. Neither EA nor PUNI induced apoptosis in normal colon CCD-112CoN cells (no chromatin condensation and no activation of caspases 3 and 9 were detected). In the case of Caco-2 cells, no specific effect can be attributed to PUNI since it was hydrolysed in the medium to yield EA, which entered into the cells and was metabolised to produce dimethyl-EA derivatives. Our study suggests that the anticarcinogenic effect of dietary ETs could be mainly due to their hydrolysis product, EA, which induced apoptosis via mitochondrial pathway in colon cancer Caco-2 cells but not in normal colon cells.
Larrosa M et al; The Journal of Nutritional Biochemistry 17 (9): 611-625 (2006)
Terminalia catappa and its major tannin component, punicalagin, have been characterized to possess antioxidative and anti-genotoxic activities. However, their effects on reactive oxygen species (ROS) mediated carcinogenesis are still unclear. In the present study, H-ras-transformed NIH3T3 cells were used to evaluate the chemopreventive effect of T. catappa water extract (TCE) and punicalagin. In the cell proliferation assay, TCE and punicalagin suppressed the proliferation of H-ras-transformed NIH3T3 cells with a dose-dependent manner but only partially affected non-transformed NIH3T3 cells proliferation. The differential cytotoxicity of TCE/punicalagin on the H-ras-transformed and non-transformed NIH3T3 cells indicated the selectivity of TCE/punicalagin against H-ras induced transformation. TCE or punicalagin treatment reduced anchorage-independent growth that could be due to a cell cycle arrest at G0/G1 phase. The intracellular superoxide level, known to modulate downstream signaling of Ras protein, was decreased by punicalagin treatments. The levels of phosphorylated JNK-1 and p38 were also decreased with punicalagin treatments. Thus, the chemopreventive effect of punicalagin against H-ras induced transformation could result from inhibition of the intracellular redox status and JNK-1/p38 activation.
PMID:16242868 Chen PS, Li JH; Toxicol Lett. 163 (1): 44-53 (2006)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
39
PharmaCompass offers a list of Punicalagin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Punicalagin manufacturer or Punicalagin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Punicalagin manufacturer or Punicalagin supplier.
PharmaCompass also assists you with knowing the Punicalagin API Price utilized in the formulation of products. Punicalagin API Price is not always fixed or binding as the Punicalagin Price is obtained through a variety of data sources. The Punicalagin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Punicalagin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Punicalagin, including repackagers and relabelers. The FDA regulates Punicalagin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Punicalagin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Punicalagin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Punicalagin supplier is an individual or a company that provides Punicalagin active pharmaceutical ingredient (API) or Punicalagin finished formulations upon request. The Punicalagin suppliers may include Punicalagin API manufacturers, exporters, distributors and traders.
click here to find a list of Punicalagin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Punicalagin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Punicalagin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Punicalagin GMP manufacturer or Punicalagin GMP API supplier for your needs.
A Punicalagin CoA (Certificate of Analysis) is a formal document that attests to Punicalagin's compliance with Punicalagin specifications and serves as a tool for batch-level quality control.
Punicalagin CoA mostly includes findings from lab analyses of a specific batch. For each Punicalagin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Punicalagin may be tested according to a variety of international standards, such as European Pharmacopoeia (Punicalagin EP), Punicalagin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Punicalagin USP).
