
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
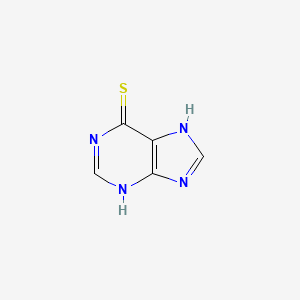

1. 1,7-dihydro-6h-purine-6-thione
2. 6 Mercaptopurine
3. 6 Mercaptopurine Monohydrate
4. 6 Thiohypoxanthine
5. 6 Thiopurine
6. 6-mercaptopurine
7. 6-mercaptopurine Monohydrate
8. 6-thiohypoxanthine
9. 6-thiopurine
10. 6h-purine-6-thione, 1,7-dihydro-
11. Bw 57 323h
12. Bw 57-323h
13. Bw 57323h
14. Leupurin
15. Mecaptopurine Anhydrous
16. Mercaptopurina Wellcome
17. Puri-nethol
18. Purimethol
19. Purinethol
1. 6-mercaptopurine
2. 50-44-2
3. Purinethol
4. Mercapurin
5. 6-thiopurine
6. Leukerin
7. Leupurin
8. Mercaleukin
9. 7h-purine-6-thiol
10. 6-thioxopurine
11. Puri-nethol
12. Purinethiol
13. Ismipur
14. Mern
15. 6-thiohypoxanthine
16. 6-mercaptopurin
17. 6-purinethiol
18. 1,9-dihydro-6h-purine-6-thione
19. Purimethol
20. 6-mp
21. Purine-6-thiol
22. 3h-purine-6-thiol
23. 6 Mp
24. 9h-purine-6-thiol
25. Mercaleukim
26. 3,7-dihydropurine-6-thione
27. 1,7-dihydro-6h-purine-6-thione
28. Hypoxanthine, Thio-
29. Mercaptopurine (6-mp)
30. Mercaptopurine Anhydrous
31. Mercaptopurin
32. Mercaptopurina
33. Mercaptopurinum
34. Merkaptopuryna
35. 6h-purine-6-thione, 1,7-dihydro-
36. Xaluprine
37. 6-merkaptopurin
38. Purine, 6-mercapto-
39. 9h-purine-6(1h)-thione
40. Purine-6(1h)-thione
41. Nci-c04886
42. 7-mercapto-1,3,4,6-tetrazaindene
43. 1h-purine, 6-mercapto-
44. Nsc 755
45. U-4748
46. 1,9-dihydropurine-6-thione
47. Purixan
48. Mercaptopurine;6-mp
49. Nsc755
50. Nsc-755
51. Mercaptopurine (inn)
52. 3,7-dihydro-6h-purine-6-thione
53. Purinethol (tn)
54. Pkk6muz20g
55. 1h-purine-6(7h)-thione.
56. Chebi:2208
57. Mercaptopurine (van)
58. Dsstox_cid_810
59. Mercaptopurin [german]
60. Merkaptopuryna [polish]
61. 6-merkaptopurin [czech]
62. Dsstox_rid_75801
63. Mercaptopurine [inn]
64. Dsstox_gsid_20810
65. Mercaptopurine (anhydrous)
66. Mercaptopurinum [inn-latin]
67. Mercaptopurina [inn-spanish]
68. Thiohypoxanthine
69. Purine-6-thiol, Monohydrate
70. Cas-50-44-2
71. 157930-11-5
72. Smr000544948
73. Mercaptopurine, 6-
74. Ccris 2761
75. Hsdb 3235
76. Sr-05000001925
77. 1194-62-3
78. Einecs 200-037-4
79. Unii-pkk6muz20g
80. Ncimech_000025
81. 9h-purin-6-yl Hydrosulfide
82. A Thiopurine
83. Mercaptopurine [usan:usp:inn]
84. 157930-13-7
85. Spectrum_000921
86. Spectrum2_000060
87. Spectrum3_000491
88. Spectrum4_000857
89. Spectrum5_000950
90. M0063
91. H-purine-6(1h)-thione
92. Azathioprine Ep Impurity B
93. Schembl3893
94. Chembl1425
95. Bspbio_001981
96. Kbiogr_001493
97. Kbiogr_002363
98. Kbioss_001401
99. Kbioss_002366
100. Mercaptopurine [hsdb]
101. Ag-670/31547064
102. Mls001066623
103. Mls001304020
104. Mls001304953
105. Mls006011869
106. Divk1c_000493
107. Spectrum1500387
108. Spbio_000219
109. 6-mercaptopurine [mi]
110. Gtpl7226
111. Schembl2790086
112. 7h-purin-6-yl Hydrosulfide #
113. 6-mercaptopurine [iarc]
114. Dtxsid0020810
115. Schembl12683725
116. Chebi:50667
117. Chebi:94796
118. Hms501i15
119. Kbio1_000493
120. Kbio2_001401
121. Kbio2_002363
122. Kbio2_003969
123. Kbio2_004931
124. Kbio2_006537
125. Kbio2_007499
126. Kbio3_001481
127. Kbio3_002842
128. 7-mercapto-1,4,6-tetrazaindene
129. Cmap_000033
130. Ninds_000493
131. 6,7-dihydro-3h-purine-6-thione
132. Hms1920l07
133. Hms2091b20
134. Hms2236l06
135. Hms3259n03
136. Hms3369m05
137. Hms3651g07
138. Hms3713n10
139. Hms3747a17
140. Hms3872n13
141. Pharmakon1600-01500387
142. Act11542
143. Discontinued, See M225450
144. Zinc4658290
145. Tox21_111158
146. Tox21_202591
147. Bdbm50423778
148. Ccg-35344
149. Ccg-39915
150. Mfcd00233552
151. Nsc759614
152. Nsc817004
153. S1305
154. Sk5357
155. Stk727062
156. Stl257085
157. 6-mercaptopurine, Analytical Standard
158. Wln: T56 Bm Dn Fn Hnj Ish
159. Akos000170222
160. Akos000275858
161. Akos005224624
162. Akos008901311
163. Akos016903205
164. Tox21_111158_1
165. Am81386
166. Ccg-266232
167. Cs-1499
168. Db01033
169. Nc00613
170. Nsc-817004
171. Idi1_000493
172. Mercaptopurine Anhydrous [who-dd]
173. Ncgc00091641-02
174. Ncgc00091641-03
175. Ncgc00091641-04
176. Ncgc00091641-16
177. Ncgc00094717-01
178. Ncgc00094717-02
179. Ncgc00094717-03
180. Ncgc00094717-05
181. Ncgc00094717-06
182. Ncgc00188973-01
183. Ncgc00188973-02
184. Ncgc00260139-01
185. Ac-11464
186. As-13109
187. Hy-13677
188. Nci60_041653
189. Smr004703503
190. Sbi-0051437.p004
191. Db-026398
192. Azathioprine Impurity B [ep Impurity]
193. Bb 0241023
194. Ft-0621175
195. Sw199090-2
196. En300-61517
197. 50m442
198. C01756
199. C02380
200. D04931
201. Ab00171799_05
202. Ab00641894-03
203. Ab00641894-04
204. Ab00641894_05
205. Ab00876276-13
206. 233d552
207. 462m721
208. 599m524
209. A828129
210. Q418529
211. Sr-05000001925-1
212. Sr-05000001925-2
213. W-105961
214. Purine Antimetabolite: Inhibits Nucleic Acid Replication
215. 1,9-dihydropurine-6-thione Discontinued, See M225450
216. Mercaptopurine; 7h-purine-6-thiol; Azathioprine Bp Impurity B
| Molecular Weight | 152.18 g/mol |
|---|---|
| Molecular Formula | C5H4N4S |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 152.01566732 g/mol |
| Monoisotopic Mass | 152.01566732 g/mol |
| Topological Polar Surface Area | 85.2 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 190 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Mercaptopurine |
| PubMed Health | Mercaptopurine (By mouth) |
| Drug Classes | Antineoplastic Agent, Antirheumatic, Cytotoxic, Gastrointestinal Agent |
| Drug Label | Mercaptopurine was synthesized and developed by Hitchings, Elion, and associates at the Wellcome Research Laboratories.Mercaptopurine, known chemically as 6H-purine-6-thione, 1,7-dihydro-, monohydrate, is an analogue of the purine bases adenine and h... |
| Active Ingredient | Mercaptopurine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Roxane; Prometheus Labs; Mylan |
| 2 of 4 | |
|---|---|
| Drug Name | Purinethol |
| PubMed Health | Mercaptopurine (By mouth) |
| Drug Classes | Antineoplastic Agent, Antirheumatic, Cytotoxic, Gastrointestinal Agent |
| Drug Label | PURINETHOL (mercaptopurine) was synthesized and developed by Hitchings, Elion, and associates at the Wellcome Research Laboratories.Mercaptopurine, known chemically as 1,7-dihydro-6H-purine-6-thione monohydrate, is an analogue of the purine bases ade... |
| Active Ingredient | Mercaptopurine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Teva |
| 3 of 4 | |
|---|---|
| Drug Name | Mercaptopurine |
| PubMed Health | Mercaptopurine (By mouth) |
| Drug Classes | Antineoplastic Agent, Antirheumatic, Cytotoxic, Gastrointestinal Agent |
| Drug Label | Mercaptopurine was synthesized and developed by Hitchings, Elion, and associates at the Wellcome Research Laboratories.Mercaptopurine, known chemically as 6H-purine-6-thione, 1,7-dihydro-, monohydrate, is an analogue of the purine bases adenine and h... |
| Active Ingredient | Mercaptopurine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Roxane; Prometheus Labs; Mylan |
| 4 of 4 | |
|---|---|
| Drug Name | Purinethol |
| PubMed Health | Mercaptopurine (By mouth) |
| Drug Classes | Antineoplastic Agent, Antirheumatic, Cytotoxic, Gastrointestinal Agent |
| Drug Label | PURINETHOL (mercaptopurine) was synthesized and developed by Hitchings, Elion, and associates at the Wellcome Research Laboratories.Mercaptopurine, known chemically as 1,7-dihydro-6H-purine-6-thione monohydrate, is an analogue of the purine bases ade... |
| Active Ingredient | Mercaptopurine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Teva |
Antimetabolites; Antimetabolites, Antineoplastic; Immunosuppressive Agents; Nucleic Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings. Mercaptopurine. Online file (MeSH, 2016). Available from, as of December 5, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Mercaptopurine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of February 1, 2017: https://clinicaltrials.gov/ct2/results?term=MERCAPTOPURINE&Search=Search
Mercaptopurine tablets are indicated for maintenance therapy of acute lymphatic (lymphocytic, lymophoblastic) leukemia as part of a combination regimen. The response to this agent depends upon the particular subclassification of acute lymphatic leukemia and the age of the patient (pediatric or adult). Mercaptopurine is not effective prophylaxis or treatment of central nervous system leukemia. Mercaptopurine is not effective in acute myelogenous leukemia, chronic lymphatic leukemia, the lymphomas (including Hodgkins Disease), or solid tumors. /Included in US product label/
NIH; DailyMed. Current Medication Information for Mercaptopurine (Updated: October 2016). Available from, as of February 6, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c3b5b8b0-bc5c-4ce9-bbdc-febba60c2658
MEDICATION (VET): Rarely used in veterinary medicine. Veterinary uses of mercaptopurine have included adjunctive therapy of lymphosarcoma, acute leukemias, and severe rheumatoid arthritis. It may have potential benefit in treating other autoimmune conditions (eg, unresponsive ulcerative colitis) as well.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 928
For more Therapeutic Uses (Complete) data for Mercaptopurine (9 total), please visit the HSDB record page.
Tumor lysis syndrome with hyperuricemia and/or hyperuricosuria may occur as a result of rapid cell lysis in patients receiving mercaptopurine as antineoplastic therapy. Prophylactic use of a xanthine oxidase inhibitor such as allopurinol may be used to minimize these adverse effects, but reduction of mercaptopurine dosage is required.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1176
VET: Like azathioprine, mercaptopurine is best avoided in cats. Additionally, with with caution in dog breeds that potentially have a low thiopurine methyltransferase (TPMT) activity (eg, giant Schnauzers).
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 929
VET: At usual doses, GI effects (nausea, anorexia, vomiting, diarrhea) are most likely seen in small animals. However, bone marrow suppression, hepatotoxicity, pancreatitis, GI (including oral) ulceration, and dermatologic reactions are, potentially, possible.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 929
Drug fever rarely has been reported in patients receiving mercaptopurine. Other causes of pyrexia, such as sepsis, should be ruled out before attributing the effect to the drug in patients with acute leukemia.103 Other infrequently occurring adverse effects of mercaptopurine include fever, headache, and excessive weakness. Oligospermia has been reported.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1177
For more Drug Warnings (Complete) data for Mercaptopurine (20 total), please visit the HSDB record page.
For remission induction and maintenance therapy of acute lymphatic leukemia.
FDA Label
Xaluprine is indicated for the treatment of acute lymphoblastic leukaemia (ALL) in adults, adolescents and children.
Mercaptopurine is one of a large series of purine analogues which interfere with nucleic acid biosynthesis and has been found active against human leukemias. It is an analogue of the purine bases adenine and hypoxanthine. It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antimetabolites, Antineoplastic
Antimetabolites that are useful in cancer chemotherapy. (See all compounds classified as Antimetabolites, Antineoplastic.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
L01BB02
L01BB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01B - Antimetabolites
L01BB - Purine analogues
L01BB02 - Mercaptopurine
Absorption
Clinical studies have shown that the absorption of an oral dose of mercaptopurine in humans is incomplete and variable, averaging approximately 50% of the administered dose. The factors influencing absorption are unknown.
Volume of Distribution
The volume of distribution exceeded that of the total body water.
/MILK/ It is not known whether mercaptopurine is distributed into milk.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1178
Mercaptopurine and its metabolites are distributed throughout total body water. The volume of distribution of mercaptopurine usually exceeds total body water content. Although the drug reportedly crosses the blood-brain barrier, CSF concentrations are not sufficient for the treatment of meningeal leukemia.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1178
Mercaptopurine is excreted in urine as unchanged drug and metabolites. In one study in adults with normal renal function, about 11% of an oral dose was recovered in the urine within 6 hours.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1179
The immunosuppressant azathioprine is increasingly being used in pregnancy. The human placenta is considered a relative barrier to the major metabolite, 6-mercaptopurine (6-MP), and likely explains the lack of proven teratogenicity in humans. The aim of this study was to determine how the human placenta restricts 6-MP transfer using the human placental perfusion model. After addition of 50 ng/mL (n=4) and 500 ng/mL (n=3) 6-MP into the maternal circulation, there was a biphasic decline in its concentration and a delay in fetal circulation appearance. Under equilibrative conditions, the fetal-to-maternal concentration ratio was >1.0 as a result of ion trapping. Binding to placental tissue and maternal pharmacokinetic parameters are the main factors that restrict placental transfer of 6-MP. Active transport is unlikely to play a significant role and drug interactions involving, or polymorphisms in, placental drug efflux transporters are not likely to put the fetus at risk of higher 6-MP exposure.
PMID:21903160 Hutson JR et al; Reprod Toxicol 32 (3): 349-53 (2011)
For more Absorption, Distribution and Excretion (Complete) data for Mercaptopurine (9 total), please visit the HSDB record page.
Hepatic. Degradation primarily by xanthine oxidase. The catabolism of mercaptopurine and its metabolites is complex. In humans, after oral administration of 35S-6-mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines. The methylthiopurines yield appreciable amounts of inorganic sulfate.
After oral administration of 35(S)-6-mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines.
NIH; DailyMed. Current Medication Information for Mercaptopurine (Updated: October 2016). Available from, as of February 6, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c3b5b8b0-bc5c-4ce9-bbdc-febba60c2658
Mercaptopurine is metabolized via 2 major pathways. Mercaptopurine is rapidly and extensively oxidized to 6-thiouric acid in the liver by the enzyme xanthine oxidase. Because xanthine oxidase is inhibited by allopurinol, concomitant use of this drug decreases the metabolism of mercaptopurine and its active metabolites and leads to toxicity. If allopurinol and mercaptopurine are used concomitantly, the dosage of mercaptopurine must be reduced to avoid toxicity. Another major catabolic pathway is thiol methylation of mercaptopurine to form the inactive metabolite methyl-6-MP. This reaction is catalyzed by the enzyme thiopurine S-methyltransferase (TPMT). Variability in TPMT activity in patients because of a genetic polymorphism in the TPMT gene causes interindividual differences in the metabolism of mercaptopurine and resulting systemic exposure to the drug and its active metabolites. Dethiolation can also occur, with large portions of the sulfur being excreted as inorganic sulfate.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1179
... In this study, we investigated the in vitro metabolism of 6-mercaptopurine (6MP) to 6-thiouric acid (6TUA) in pooled human liver cytosol. We discovered that 6MP is metabolized to 6TUA through sequential metabolism via the 6-thioxanthine (6TX) intermediate. The role of human AO and XO in the metabolism of 6MP was established using the specific inhibitors raloxifene and febuxostat. Both AO and XO were involved in the metabolism of the 6TX intermediate, whereas only XO was responsible for the conversion of 6TX to 6TUA. These findings were further confirmed using purified human AO and Escherichia coli lysate containing expressed recombinant human XO. Xanthine dehydrogenase (XDH), which belongs to the family of xanthine oxidoreductases and preferentially reduces nicotinamide adenine dinucleotide (NAD(+)), was shown to contribute to the overall production of the 6TX intermediate as well as the final product 6TUA in the presence of NAD(+) in human liver cytosol. In conclusion, we present evidence that three enzymes, AO, XO, and XDH, contribute to the production of 6TX intermediate, whereas only XO and XDH are involved in the conversion of 6TX to 6TUA in pooled HLC.
PMID:24824603 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4109211 Choughule KV et al; Drug Metab Dispos 42 (8): 1334-40 (2014)
The thiopurine antimetabolites, 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG) are inactive pro-drugs that require intracellular metabolism for activation to cytotoxic metabolites. Thiopurine methyltransferase (TPMT) is one of the most important enzymes in this process metabolizing both 6-MP and 6-TG to different methylated metabolites including methylthioinosine monophosphate (meTIMP) and methylthioguanosine monophosphate (meTGMP), respectively, with different suggested pharmacological and cytotoxic properties. While meTIMP is a potent inhibitor of de novo purine synthesis (DNPS) and significantly contributes to the cytotoxic effects of 6-MP, meTGMP, does not add much to the effects of 6-TG, and the cytotoxicity of 6-TG seems to be more dependent on incorporation of thioguanine nucleotides (TGNs) into DNA rather than inhibition of DNPS. In order to investigate the role of TPMT in metabolism and thus, cytotoxic effects of 6-MP and 6-TG, we knocked down the expression of the gene encoding the TPMT enzyme using specifically designed small interference RNA (siRNA) in human MOLT4 leukemia cells. The knock-down was confirmed at RNA, protein, and enzyme function levels. Apoptosis was determined using annexin V and propidium iodide staining and FACS analysis. The results showed a 34% increase in sensitivity of MOLT4 cells to 1 uM 6-TG after treatment with TPMT-targeting siRNA, as compared to cells transfected with non-targeting siRNA, while the sensitivity of the cells toward 6-MP was not affected significantly by down-regulation of the TPMT gene. This differential contribution of the enzyme TPMT to the cytotoxicity of the two thiopurines is probably due to its role in formation of the meTIMP, the cytotoxic methylated metabolite of 6-MP, while in case of 6-TG methylation by TPMT substantially deactivates the drug.
PMID:23811272 Karim H et al; Biochem Biophys Res Commun 437 (2): 280-6 (2013)
6-Thiouric acid is the major metabolite of 6-mercaptopurine and is formed from this drug by the action of xanthine oxidase.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 257 (1981)
Triphasic: 45 minutes, 2.5 hours, and 10 hours.
Following IV administration of mercaptopurine (an IV preparation of the drug currently is not commercially available in the US), the elimination half-life of the drug is reportedly 21 minutes in pediatric patients and 47 minutes in adults.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 1178
After an intravenous dose, the half-life of the drug in plasma is relatively short (about 50 minutes) due to uptake by cells, renal excretion, and rapid metabolic degradation.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1235
After iv administration of 6-mercaptopurine, the half-Iie for disappearance from the blood was about 9 min in rats and 14 min in mice.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 257 (1981)
Mercaptopurine competes with hypoxanthine and guanine for the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). TIMP inhibits several reactions that involve inosinic acid (IMP), such as the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). Upon methylation, TIMP forms 6-methylthioinosinate (MTIMP) which inhibits glutamine-5-phosphoribosylpyrophosphate amidotransferase in addition to TIMP. Glutamine-5-phosphoribosylpyrophosphate amidotransferase is the first enzyme unique to the _de novo_ pathway for purine ribonucleotide synthesis. According to experimental findings using radiolabeled mercaptopurine, mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. In comparison, some mercaptopurine may be converted to nucleotide derivatives of 6-thioguanine (6-TG) via actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase that convert TIMP to thioguanylic acid (TGMP).
The pathogenesis of several neurodegenerative diseases often involves the microglial activation and associated inflammatory processes. Activated microglia release pro-inflammatory factors that may be neurotoxic. 6-Mercaptopurine (6-MP) is a well-established immunosuppressive drug. Common understanding of their immunosuppressive properties is largely limited to peripheral immune cells. However, the effect of 6-MP in the central nervous system, especially in microglia in the context of neuroinflammation is, as yet, unclear. Tumor necrosis factor-alpha (TNF-a) is a key cytokine of the immune system that initiates and promotes neuroinflammation. The present study aimed to investigate the effect of 6-MP on TNF-a production by microglia to discern the molecular mechanisms of this modulation. Lipopolysaccharide (LPS) was used to induce an inflammatory response in cultured primary microglia or murine BV-2 microglial cells. Released TNF-a was measured by enzyme-linked immunosorbent assay (ELISA). Gene expression was determined by real-time reverse transcription polymerase chain reaction (RT-PCR). Signaling molecules were analyzed by western blotting, and activation of NF-kB was measured by ELISA-based DNA binding analysis and luciferase reporter assay. Chromatin immunoprecipitation (ChIP) analysis was performed to examine NF-kB p65 and coactivator p300 enrichments and histone modifications at the endogenous TNF-a promoter. Treatment of LPS-activated microglia with 6-MP significantly attenuated TNF-a production. In 6-MP pretreated microglia, LPS-induced MAPK signaling, I?B-a degradation, NF-kB p65 nuclear translocation, and in vitro p65 DNA binding activity were not impaired. However, 6-MP suppressed transactivation activity of NF-?B and TNF-a promoter by inhibiting phosphorylation and acetylation of p65 on Ser276 and Lys310, respectively. ChIP analyses revealed that 6-MP dampened LPS-induced histone H3 acetylation of chromatin surrounding the TNF-a promoter, ultimately leading to a decrease in p65/coactivator-mediated transcription of TNF-a gene. Furthermore, 6-MP enhanced orphan nuclear receptor Nur77 expression. Using RNA interference approach, we further demonstrated that Nur77 upregulation contribute to 6-MP-mediated inhibitory effect on TNF-a production. Additionally, 6-MP also impeded TNF-a mRNA translation through prevention of LPS-activated PI3K/Akt/mTOR signaling cascades. These results suggest that 6-MP might have a therapeutic potential in neuroinflammation-related neurodegenerative disorders through downregulation of microglia-mediated inflammatory processes.
PMID:27075886 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4831152 Huang HY et al; J Neuroinflammation 13 (1): 78 (2016)
Mercaptopurine (6-MP) competes with hypoxanthine and guanine for the enzyme hyphoxanthine-guanine phosphoribosyltransferase (HGPRTase) and is itself converted to thioinosinic acid (TIMP). This intracellular nucleotide inhibits several reactions involving inosinic acid (IMP), including the conversion of IMP to xanthylic acid (XMP) and the conversion of IMP to adenylic acid (AMP) via adenylosuccinate (SAMP). In addition, 6-methylthioinosinate (MTIMP) is formed by the methylation of TIMP. Both TIMP and MTIMP have been reported to inhibit glutamine-5-phosphoribosylpyrophosphate amidotransferase, the first enzyme unique to the de novo pathway for purine ribonucleotide synthesis. Experiments indicate that radiolabeled mercaptopurine may be recovered from the DNA in the form of deoxythioguanosine. Some mercaptopurine is converted to nucleotide derivatives of 6-thioguanine (6-TG) by the sequential actions of inosinate (IMP) dehydrogenase and xanthylate (XMP) aminase, converting TIMP to thioguanylic acid (TGMP). Animal tumors that are resistant to mercaptopurine often have lost the ability to convert mercaptopurine to TIMP. However, it is clear that resistance to mercaptopurine may be acquired by other means as well, particularly in human leukemias. It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death.
NIH; DailyMed. Current Medication Information for Mercaptopurine (Updated: October 2016). Available from, as of February 6, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c3b5b8b0-bc5c-4ce9-bbdc-febba60c2658
Inflammatory bowel disease is characterized by chronic intestinal inflammation. Azathioprine and its metabolite 6-mercaptopurine (6-MP) are effective immunosuppressive drugs that are widely used in patients with inflammatory bowel disease. ... Azathioprine and 6-MP have been shown to affect small GTPase Rac1 in T cells and endothelial cells, whereas the effect on macrophages and gut epithelial cells is unknown. Macrophages (RAW cells) and gut epithelial cells (Caco-2 cells) were activated by cytokines and the effect on Rac1 signaling was assessed in the presence or absence of 6-MP. Rac1 is activated in macrophages and epithelial cells, and treatment with 6-MP resulted in Rac1 inhibition. In macrophages, interferon-gamma induced downstream signaling through c-Jun-N-terminal Kinase (JNK) resulting in inducible nitric oxide synthase (iNOS) expression. iNOS expression was reduced by 6-MP in a Rac1-dependent manner. In epithelial cells, 6-MP efficiently inhibited tumor necrosis factor-a-induced expression of the chemokines CCL2 and interleukin-8, although only interleukin-8 expression was inhibited in a Rac1-dependent manner. In addition, activation of the transcription factor STAT3 was suppressed in a Rac1-dependent fashion by 6-MP, resulting in reduced proliferation of the epithelial cells due to diminished cyclin D1 expression. These data demonstrate that 6-MP affects macrophages and gut epithelial cells beneficially, in addition to T cells and endothelial cells. Furthermore, mechanistic insight is provided to support development of Rac1-specific inhibitors for clinical use in inflammatory bowel disease.
PMID:25029617 Marinkovic G et al; Inflamm Bowel Dis 20 (9): 1487-95 (2014)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
77
PharmaCompass offers a list of Mercaptopurine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Mercaptopurine manufacturer or Mercaptopurine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Mercaptopurine manufacturer or Mercaptopurine supplier.
PharmaCompass also assists you with knowing the Mercaptopurine API Price utilized in the formulation of products. Mercaptopurine API Price is not always fixed or binding as the Mercaptopurine Price is obtained through a variety of data sources. The Mercaptopurine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Purinethol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Purinethol, including repackagers and relabelers. The FDA regulates Purinethol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Purinethol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Purinethol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Purinethol supplier is an individual or a company that provides Purinethol active pharmaceutical ingredient (API) or Purinethol finished formulations upon request. The Purinethol suppliers may include Purinethol API manufacturers, exporters, distributors and traders.
click here to find a list of Purinethol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Purinethol DMF (Drug Master File) is a document detailing the whole manufacturing process of Purinethol active pharmaceutical ingredient (API) in detail. Different forms of Purinethol DMFs exist exist since differing nations have different regulations, such as Purinethol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Purinethol DMF submitted to regulatory agencies in the US is known as a USDMF. Purinethol USDMF includes data on Purinethol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Purinethol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Purinethol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Purinethol Drug Master File in Japan (Purinethol JDMF) empowers Purinethol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Purinethol JDMF during the approval evaluation for pharmaceutical products. At the time of Purinethol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Purinethol suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Purinethol Drug Master File in Korea (Purinethol KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Purinethol. The MFDS reviews the Purinethol KDMF as part of the drug registration process and uses the information provided in the Purinethol KDMF to evaluate the safety and efficacy of the drug.
After submitting a Purinethol KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Purinethol API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Purinethol suppliers with KDMF on PharmaCompass.
A Purinethol CEP of the European Pharmacopoeia monograph is often referred to as a Purinethol Certificate of Suitability (COS). The purpose of a Purinethol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Purinethol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Purinethol to their clients by showing that a Purinethol CEP has been issued for it. The manufacturer submits a Purinethol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Purinethol CEP holder for the record. Additionally, the data presented in the Purinethol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Purinethol DMF.
A Purinethol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Purinethol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Purinethol suppliers with CEP (COS) on PharmaCompass.
A Purinethol written confirmation (Purinethol WC) is an official document issued by a regulatory agency to a Purinethol manufacturer, verifying that the manufacturing facility of a Purinethol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Purinethol APIs or Purinethol finished pharmaceutical products to another nation, regulatory agencies frequently require a Purinethol WC (written confirmation) as part of the regulatory process.
click here to find a list of Purinethol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Purinethol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Purinethol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Purinethol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Purinethol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Purinethol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Purinethol suppliers with NDC on PharmaCompass.
Purinethol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Purinethol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Purinethol GMP manufacturer or Purinethol GMP API supplier for your needs.
A Purinethol CoA (Certificate of Analysis) is a formal document that attests to Purinethol's compliance with Purinethol specifications and serves as a tool for batch-level quality control.
Purinethol CoA mostly includes findings from lab analyses of a specific batch. For each Purinethol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Purinethol may be tested according to a variety of international standards, such as European Pharmacopoeia (Purinethol EP), Purinethol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Purinethol USP).
