
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
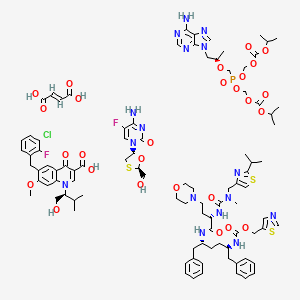

1. Elvitegravir Cobicistat Emtricitabine Tenofovir Disoproxil Fumarate Drug Combination
2. Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate
3. Elvitegravir, Cobicistat, Emtricitabine, And Tenofovir Disoproxil Fumarate Drug Combination
4. Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Disoproxil Fumarate Drug Combination
5. Elvitegravir-cobicistat-emtricitabine-tenofovir Disoproxil Fumarate Drug Combination
6. Genvoya
7. Pill, Quad
8. Quad Pill
1. Cobicistat & Elvitegravir & Emtricitabine & Tenofovir Disoproxil Fumarate
2. Cobicistat + Elvitegravir + Emtricitabine + Tenofovir Disoproxil Fumarate
3. Cobicistat And Elvitegravir And Emtricitabine And Tenofovir Disoproxil Fumarate
4. Quad
5. Quad Pill
6. Quad Tablet
7. Evg-cobi-ftc-tdf
8. Cobicistat, Elvitegravir, Emtricitabine, Tenofovir
9. Cobicistat / Elvitegravir / Emtricitabine / Tenofovir Disoproxil
10. Elvitegravir-cobicistat-emtricitabine-tenofovir Disoproxil Fumarate
11. Cobicistat / Elvitegravir / Emtricitabine / Tenofovir Disoproxil Fumarate
12. Cobicistat Mixture With Elvitegravir And Emtricitabine And Tenofovir Disoproxil
13. Cobicistat Mixture With Elvitegravir, Emtricitabine And Tenofovir Disoproxil Fumarate
14. Elvitegravir Mixture With Cobicistat And Emtricitabine And Tenofovir Disoproxil Fumarate
15. Elvitegravir, Cobicistat, Emtricitabine, Tenofovir Disoproxil Fumarate Drug Combination
16. 1430117-57-9
| Molecular Weight | 2106.7 g/mol |
|---|---|
| Molecular Formula | C94H120ClF2N16O27PS3 |
| Hydrogen Bond Donor Count | 10 |
| Hydrogen Bond Acceptor Count | 40 |
| Rotatable Bond Count | 48 |
| Exact Mass | 2105.7098720 g/mol |
| Monoisotopic Mass | 2104.7065172 g/mol |
| Topological Polar Surface Area | 655 Ų |
| Heavy Atom Count | 144 |
| Formal Charge | 0 |
| Complexity | 3010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
| 1 of 2 | |
|---|---|
| Drug Name | Stribild |
| PubMed Health | Elvitegravir/Cobicistat/Emtricitabine/Tenofovir (By mouth) |
| Drug Classes | Anti-Infective Agent, Antiviral, Reverse Transcriptase Inhibitor Combination |
| Drug Label | STRIBILD is a fixed-dose combination tablet containing elvitegravir, cobicistat, emtricitabine, and tenofovir DF for oral administration.Elvitegravir is an HIV-1 integrase strand transfer inhibitor.Cobicistat is a mechanism-based inhibitor of cytochr... |
| Active Ingredient | elvitegravir; Cobicistat; tenofovir disoproxil fumarate; emtricitabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 150mg; 300mg |
| Market Status | Prescription |
| Company | Gilead Sciences |
| 2 of 2 | |
|---|---|
| Drug Name | Stribild |
| PubMed Health | Elvitegravir/Cobicistat/Emtricitabine/Tenofovir (By mouth) |
| Drug Classes | Anti-Infective Agent, Antiviral, Reverse Transcriptase Inhibitor Combination |
| Drug Label | STRIBILD is a fixed-dose combination tablet containing elvitegravir, cobicistat, emtricitabine, and tenofovir DF for oral administration.Elvitegravir is an HIV-1 integrase strand transfer inhibitor.Cobicistat is a mechanism-based inhibitor of cytochr... |
| Active Ingredient | elvitegravir; Cobicistat; tenofovir disoproxil fumarate; emtricitabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 150mg; 300mg |
| Market Status | Prescription |
| Company | Gilead Sciences |
Treatment of human immunodeficiency virus 1 (HIV 1) infection in adults aged 18 years and over who are antiretroviral treatment-nave or are infected with HIV 1 without known mutations associated with resistance to any of the three antiretroviral agents in Stribild.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
J05AR09
Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?