
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Listed Dossiers
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
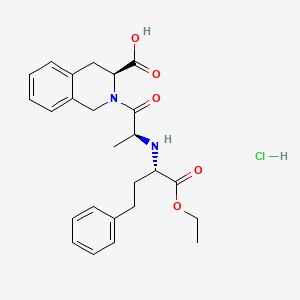

1. 2-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid
2. Accupril
3. Ci 906
4. Ci-906
5. Pd 109,452 2
6. Pd 109452 2
7. Pd 109452-2
8. Pd 1094522
9. Pd-109,452-2
10. Quinapril
1. 82586-55-8
2. Accupril
3. Quinapril Hcl
4. Accuprin
5. Korec
6. Acequin
7. Quinazil
8. Hemokvin
9. Lidaltrin
10. Acuitel
11. Ectren
12. Conan
13. Quinapril (hydrochloride)
14. Accupro
15. Ci-906
16. Quinapril.hcl
17. Ci906
18. Ci 906
19. Quinaprilhydrochloride
20. Quinapril Hydrochloride
21. Nsc-758222
22. Chebi:8714
23. Quinapril Hydrochloride (accupril)
24. 33067b3n2m
25. (3s)-2-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,4-dihydro-1h-isoquinoline-3-carboxylic Acid;hydrochloride
26. (s)-2-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)-l-alanyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid Hydrochloride
27. (s)-2-((s)-n-((s)-1-carboxy-3-phenylpropyl)alanyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid, 1-ethyl Ester, Monohydrochloride
28. Accupron
29. Acuprel
30. Continucor
31. Koretic
32. Asig
33. Dsstox_cid_1221
34. Dsstox_rid_76019
35. Dsstox_gsid_21221
36. (s)-2-((s)-2-((s)-1-ethoxy-1-oxo-4-phenylbutan-2-ylamino)propanoyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid Hydrochloride
37. Cas-82586-55-8
38. Quinapril Hydrochloride [usan]
39. Hsdb 7046
40. Pd 109452-2
41. Unii-33067b3n2m
42. Quinapril N-oxide
43. Ncgc00094997-01
44. Accupril (tn)
45. Quinapril Hydrochloride [usan:usp]
46. Mfcd00889215
47. Quinapril Hydrochloride;
48. Schembl41404
49. Mls001076683
50. Quinapril Hydrochloride ,(s)
51. Spectrum1503076
52. Quinapril Hydrochloride- Bio-x
53. Quinapril For System Suitability
54. Chembl1201011
55. Dtxsid3021221
56. Quinapril For Peak Identification
57. Hms1922c15
58. Pharmakon1600-01503076
59. Tetrahydroisoquinoline-3-carboxylic
60. Hy-b0477
61. 2-ylamino)propanoyl)-1,2,3,4-
62. Quinapril Hydrochloride [mi]
63. Tox21_111381
64. Tox21_200390
65. Ccg-39543
66. Nsc758222
67. Quinapril Hydrochloride (jp17/usp)
68. Quinapril Hydrochloride [jan]
69. S2581
70. Quinapril Hydrochloride [hsdb]
71. Akos015888532
72. Pd-109452-2
73. Tox21_111381_1
74. Ac-1655
75. Nc00722
76. Nsc 758222
77. Quinapril Hydrochloride [mart.]
78. Quinapril Hydrochloride [vandf]
79. Quinapril Hydrochloride [usp-rs]
80. Quinapril Hydrochloride [who-dd]
81. Ncgc00167962-06
82. Ncgc00257944-01
83. (3s)-2-(n-{(1s)-1-[(ethyloxy)carbonyl]-3-phenylpropyl}-l-alanyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid Hydrochloride
84. (3s)-2-[(2s)-2-{[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid Hydrochloride
85. 3-isoquinolinecarboxylic Acid, 1,2,3,4-tetrahydro-2-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-, Monohydrochloride, (3s-(2(r*(r*)),3r*))-
86. 3-isoquinolinecarboxylic Acid, 2-(2-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)-1-oxopropyl)-1,2,3,4-tetrahydro-, Monohydrochloride, (3s-(2(r*(r*)),3r*))
87. As-14026
88. Bq164301
89. Smr000499582
90. Pd-109522
91. Pd109452-2
92. Quinapril Hydrochloride [orange Book]
93. Sw199393-2
94. Quinapril Hydrochloride [ep Monograph]
95. Quinapril Hydrochloride [usp Impurity]
96. C07340
97. D00459
98. F20594
99. Quinapril Hydrochloride [usp Monograph]
100. Quinapril Hydrochloride, >=98% (hplc), Solid
101. 586q558
102. A840381
103. Accuretic Component Quinapril Hydrochloride
104. Quinaretic Component Quinapril Hydrochloride
105. Sr-01000765404
106. Sr-01000765404-2
107. Q27108138
108. Quinapril Hydrochloride Component Of Accuretic
109. Quinapril Hydrochloride Component Of Quinaretic
110. (s)-2-((s)-2-((s)-1-ethoxy-1-oxo-4-phenylbutan-
111. Quinapril Hydrochloride, European Pharmacopoeia (ep) Reference Standard
112. Quinapril Hydrochloride, United States Pharmacopeia (usp) Reference Standard
113. Quinapril For Peak Identification, European Pharmacopoeia (ep) Reference Standard
114. Quinapril For System Suitability, European Pharmacopoeia (ep) Reference Standard
115. Quinapril Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
116. (3s)-2-{(2s)-2-[(1s)-1-ethoxycarbonyl-3-phenylpropylamino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid Hydrochloride
117. (3s)-2-{n-[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]-l-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid Hydrochloride
118. 2-(2-((1-ethoxycarbonyl-3-phenylpropyl)amino)-1-oxopropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic Acid Monohydrochloride(3s-(2(r*(r*)),3r*)
| Molecular Weight | 475.0 g/mol |
|---|---|
| Molecular Formula | C25H31ClN2O5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | 474.1921498 g/mol |
| Monoisotopic Mass | 474.1921498 g/mol |
| Topological Polar Surface Area | 95.9 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 648 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Accupril |
| PubMed Health | Quinapril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | ACCUPRIL (quinapril hydrochloride) is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.Quinapril hydrochloride is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2... |
| Active Ingredient | Quinapril hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 40mg base; eq 5mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 2 of 6 | |
|---|---|
| Drug Name | Accuretic |
| PubMed Health | Quinapril/Hydrochlorothiazide (By mouth) |
| Drug Classes | ACE Inhibitor/Thiazide Combination, Antihypertensive, Cardiovascular Agent |
| Active Ingredient | Hydrochlorothiazide; quinapril hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; eq 20mg base; eq 10mg base; 12.5mg |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 3 of 6 | |
|---|---|
| Drug Name | Quinapril hydrochloride |
| Drug Label | Quinapril hydrochloride is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.Quinapril hydrochloride is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2-[[1-(ethox... |
| Active Ingredient | Quinapril hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 20mg base; eq 40mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Ranbaxy; Actavis Labs Fl; Teva; Aurobindo Pharma; Sun Pharm Inds; Lupin; Invagen Pharms; Mylan |
| 4 of 6 | |
|---|---|
| Drug Name | Accupril |
| PubMed Health | Quinapril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | ACCUPRIL (quinapril hydrochloride) is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.Quinapril hydrochloride is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2... |
| Active Ingredient | Quinapril hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 40mg base; eq 5mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 5 of 6 | |
|---|---|
| Drug Name | Accuretic |
| PubMed Health | Quinapril/Hydrochlorothiazide (By mouth) |
| Drug Classes | ACE Inhibitor/Thiazide Combination, Antihypertensive, Cardiovascular Agent |
| Active Ingredient | Hydrochlorothiazide; quinapril hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; eq 20mg base; eq 10mg base; 12.5mg |
| Market Status | Prescription |
| Company | Pfizer Pharms |
| 6 of 6 | |
|---|---|
| Drug Name | Quinapril hydrochloride |
| Drug Label | Quinapril hydrochloride is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.Quinapril hydrochloride is chemically described as [3S-[2[R*(R*)], 3R*]]-2-[2-[[1-(ethox... |
| Active Ingredient | Quinapril hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 20mg base; eq 40mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Ranbaxy; Actavis Labs Fl; Teva; Aurobindo Pharma; Sun Pharm Inds; Lupin; Invagen Pharms; Mylan |
... /Quinapril/ has proven to be very useful for the treatment of hypertension ... . /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 893
The angiotensin converting enzyme (ACE) inhibitors appear to confer a special advantage in the treatment of patients with diabetes, slowing the development of diabetic glomerulopathy. They also have been shown to be effective in slowing the progression of other forms of chronic renal disease, such as glomerulosclerosis, & many of these patients also have hypertension. An ACE inhibitor is probably the preferred initial agent in the treatment of hypertensive patients with left ventricular hypertrophy. Patients with hypertension & ischemic heart disease are candidates for treatment with ACE inhibitors; this includes treatment in the immediate post-myocardial infarction period which has been shown to lead to improved ventricular function & reduced morbidity & mortality. /ACE inhibitors/ /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 893
The combination of ... quinapril and hydrochlorothiazide is indicated in the treatment of hypertension. Fixed-dosage combinations generally are not recommended for initial therapy, but are utilized in maintenance therapy after the required dose is established in order to increase convenience, economy, and patient compliance. /Included in US product labeling/ /Salt not specified/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 215
Angiotensin converting enzyme (ACE) inhibitor /Salt not specified/
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1441
For more Therapeutic Uses (Complete) data for QUINAPRIL HYDROCHLORIDE (6 total), please visit the HSDB record page.
Reduce dose ... in patients with serum creatinine > or =221 umol/L (2.5 mg/dL). /ACE Inhibitors; from table/ /Salt not specified/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 553
May cause hyperkalemia in patients with renal impairment or in those receiving potassium-sparing agents. /ACE Inhibitors; from table/ /Salt not specified/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 553
Can cause acute renal failure in patients with severe bilateral renal artery stenosis or severe stenosis in artery to solitary kidney. /ACE Inhibitors; from table/ /Salt not specified/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 553
Conversion of quinapril to quinaprilat is reduced in patients with diminished liver function. /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 824
For more Drug Warnings (Complete) data for QUINAPRIL HYDROCHLORIDE (12 total), please visit the HSDB record page.
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
Absorption (bioavailability) of quinapril is 60%; time to peak serum concn is 2 hr; half-life (elimination) is 2 hr; protein binding is 97%; metabolism is in the liver. /from table/ /Salt not specified/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 557
Quinapril is rapidly absorbed (peak concns are achieved in 1 hr, but the peak may be delayed after food), & its rate but not extent of oral absorption (60%) may be reduced by food. Quinapril is metabolized to quinaprilat & to other minor metabolites, & quinaprilat is excreted in the urine (61%) & the feces (37%). Peak concns of quinaprilat in plasma are achieved in about 2 hr. Conversion of quinapril to quinaprilat is reduced in patients with diminished liver function. The initial half-life of quinaprilat is about 2 hr; a prolonged terminal half-life of about 25 hr may be due to high-affinity binding of the drug to tissue ACE. /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 823
Cleavage of the ester moiety by hepatic esterases transforms quinapril hydrochloride, a prodrug, into quinaprilat, an ACE inhibitor that in vitro is about as potent as benazeprilat. ... Quinapril is metabolized to quinaprilat & to other minor metabolites ... .
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 823
The initial half-life of /the metabolite/ quinaprilat is about 2 hr; a prolonged terminal half-life of about 25 hr may be due to high-affinity binding of the drug to tissue ACE. /Salt not specified/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 824
Block formation of angiotensin II, promoting vasodilation & decreased aldosterone; also increased bradykinin & vasodilatory prostaglandins. /ACE Inhibitors; from table/ /Salt not specified/
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 553
Quinapril is deesterified to the principal metabolite, quinaprilat, which is an inhibitor of ACE activity in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor, angiotensin II. The effect of quinapril in hypertension and in congestive heart failure (CHF) appears to result primarily from the inhibition of circulating and tissue ACE activity, thereby reducing angiotensin II formation. Quinapril inhibits the elevation in blood pressure caused by iv administered angiotensin I, but has no effect on the pressor response to angiotensin II, norepinephrine or epinephrine. Angiotensin II also stimulates the secretion of aldosterone from the adrenal cortex, thereby facilitating renal sodium and fluid reabsorption. Reduced aldosterone secretion by quinapril may result in a small incr in serum potassium. In controlled hypertension trials, treatment with ACCUPRIL alone resulted in mean increases in potassium of 0.07 mmol/L ... . Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity (PRA). /Salt not specified/
Medical Economics Co; Physicians Desk Reference 56th ed p.2611 (2002)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
39
PharmaCompass offers a list of Quinapril API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Quinapril manufacturer or Quinapril supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Quinapril manufacturer or Quinapril supplier.
PharmaCompass also assists you with knowing the Quinapril API Price utilized in the formulation of products. Quinapril API Price is not always fixed or binding as the Quinapril Price is obtained through a variety of data sources. The Quinapril Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Quinapril hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Quinapril hydrochloride, including repackagers and relabelers. The FDA regulates Quinapril hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Quinapril hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Quinapril hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Quinapril hydrochloride supplier is an individual or a company that provides Quinapril hydrochloride active pharmaceutical ingredient (API) or Quinapril hydrochloride finished formulations upon request. The Quinapril hydrochloride suppliers may include Quinapril hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Quinapril hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Quinapril hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Quinapril hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of Quinapril hydrochloride DMFs exist exist since differing nations have different regulations, such as Quinapril hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Quinapril hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. Quinapril hydrochloride USDMF includes data on Quinapril hydrochloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Quinapril hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Quinapril hydrochloride suppliers with USDMF on PharmaCompass.
A Quinapril hydrochloride CEP of the European Pharmacopoeia monograph is often referred to as a Quinapril hydrochloride Certificate of Suitability (COS). The purpose of a Quinapril hydrochloride CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Quinapril hydrochloride EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Quinapril hydrochloride to their clients by showing that a Quinapril hydrochloride CEP has been issued for it. The manufacturer submits a Quinapril hydrochloride CEP (COS) as part of the market authorization procedure, and it takes on the role of a Quinapril hydrochloride CEP holder for the record. Additionally, the data presented in the Quinapril hydrochloride CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Quinapril hydrochloride DMF.
A Quinapril hydrochloride CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Quinapril hydrochloride CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Quinapril hydrochloride suppliers with CEP (COS) on PharmaCompass.
A Quinapril hydrochloride written confirmation (Quinapril hydrochloride WC) is an official document issued by a regulatory agency to a Quinapril hydrochloride manufacturer, verifying that the manufacturing facility of a Quinapril hydrochloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Quinapril hydrochloride APIs or Quinapril hydrochloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Quinapril hydrochloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Quinapril hydrochloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Quinapril hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Quinapril hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Quinapril hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Quinapril hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Quinapril hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Quinapril hydrochloride suppliers with NDC on PharmaCompass.
Quinapril hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Quinapril hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Quinapril hydrochloride GMP manufacturer or Quinapril hydrochloride GMP API supplier for your needs.
A Quinapril hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Quinapril hydrochloride's compliance with Quinapril hydrochloride specifications and serves as a tool for batch-level quality control.
Quinapril hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Quinapril hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Quinapril hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Quinapril hydrochloride EP), Quinapril hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Quinapril hydrochloride USP).
