
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Adaquin
2. Apo Quinidine
3. Apo-quinidine
4. Chinidin
5. Quincardine
6. Quinidex
7. Quinidine Sulfate
8. Quinora
9. Sulfate, Quinidine
1. 56-54-2
2. (+)-quinidine
3. Conquinine
4. Pitayine
5. Conchinin
6. Chinidin
7. (8r,9s)-quinidine
8. Quinidex
9. Beta-quinine
10. (9s)-6'-methoxycinchonan-9-ol
11. Cin-quin
12. Kinidin
13. Quinora
14. Cinchonan-9-ol, 6'-methoxy-, (9s)-
15. Conchinine
16. Chinidinum
17. Quinidina
18. Chinidine
19. .beta.-quinidine
20. Quinaglute
21. Quiniduran
22. Auriquin
23. Alpha-(6-methoxy-4-quinolyl)-5-vinyl-2-quinuclidinemethanol
24. (s)-[(2r,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl](6-methoxyquinolin-4-yl)methanol
25. Quinidine Sulfate
26. Chebi:28593
27. Quinicardine
28. Chembl1294
29. Quinact
30. Quinalan
31. Quinatime
32. (1s)-(6-methoxyquinolin-4-yl)((2r,4s,5r)-5-vinylquinuclidin-2-yl)methanol
33. Itx08688jl
34. 6-methoxy-alpha-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol
35. Beta-quinidine
36. (3'.alpha., 9s)-6'-methoxycinchonan-9-ol
37. (s)-[(2r,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol
38. Tcmdc-131239
39. Nci-c56246
40. Mfcd00135581
41. (r)-(6-methoxyquinolin-4-yl)((3s,4r,7s)-3-vinylquinuclidin-7-yl)methanol
42. (s)-(6-methoxy-4-quinolyl)-[(2r,4s,5r)-5-vinylquinuclidin-2-yl]methanol
43. (s)-(6-methoxyquinolin-4-yl)((1s,2r,4s,5r)-5-vinylquinuclidin-2-yl)methanol
44. (s)-[(4s,5r,7r)-5-ethenyl-1-azabicyclo[2.2.2]octan-7-yl]-(6-methoxyquinolin-4-yl)methanol
45. Chinidin [german]
46. Smr000857275
47. Quinidine [ban:nf]
48. Unii-itx08688jl
49. Quinindine
50. Ccris 672
51. Hsdb 225
52. (8r,9s)-6'-methoxycinchonan-9-ol
53. (s)-(6-methoxyquinolin-4-yl)((2r,5r)-5-vinylquinuclidin-2-yl)methanol
54. (s)-(6-methoxy-quinolin-4-yl)-((2r,5r)-5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methanol
55. Qdn
56. Einecs 200-279-0
57. Quinidine, Anhydrous
58. Quinidine [mi]
59. Quinidine [hsdb]
60. Prestwick3_000280
61. Quinidine [vandf]
62. Bmse000511
63. Epitope Id:141803
64. Quinidine [mart.]
65. Quinidine [who-dd]
66. Schembl15943
67. Bspbio_000160
68. Mls001335913
69. Mls001335914
70. Mls002548869
71. Bpbio1_000176
72. Gtpl2342
73. Dtxsid4023549
74. Schembl17537608
75. Hms2234l10
76. Hms3259o09
77. 101143-86-6
78. Act09863
79. Hy-b1751
80. Zinc3831405
81. (9s)-6-methoxy-alpha-(5-vinyl-2-quinuclidinyl)-4-quinolinemethanol
82. Alpha-(6-methoxy-4-quinolyl)-5-vinyl-2-quinuclidinemethanol (9s)-
83. Bdbm50121975
84. Akos015920101
85. Ccg-256507
86. Cs-7812
87. Db00908
88. Nc00478
89. Sdccgmls-0066600.p001
90. Ncgc00091231-01
91. Ncgc00091231-02
92. Ncgc00091231-03
93. Ncgc00091231-18
94. As-30538
95. Quinine Sulfate Impurity A [who-ip]
96. Ab00514657
97. Quinine Bisulfate Impurity A [who-ip]
98. Ab01562940_01
99. Quinine Sulfate Impurity A [ep Impurity]
100. Q412496
101. W-109256
102. Brd-k59632282-052-01-5
103. Brd-k59632282-052-02-3
104. Brd-k70799801-311-02-7
105. Quinine Hydrochloride Impurity A [ep Impurity]
106. Quinidine, Crystallized, >=98.0% (dried Material, Nt)
107. Quinine Bisulfate Heptahydrate Impurity A [who-ip]
108. (s)-((2s,4s,5r)-5-ethenyl-1-azabicyclo(2.2.2)oct-2-yl)(6-methoxyquinolin-4-yl)methanol
| Molecular Weight | 324.4 g/mol |
|---|---|
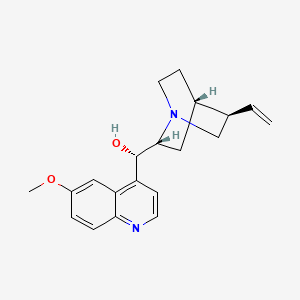
| Molecular Formula | C20H24N2O2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 324.183778013 g/mol |
| Monoisotopic Mass | 324.183778013 g/mol |
| Topological Polar Surface Area | 45.6 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 457 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Quinidine sulfate |
| Drug Label | Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the -isomer of quinine, and its molecular weight is 324.43. dQuinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,... |
| Active Ingredient | Quinidine sulfate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 200mg; 300mg |
| Market Status | Prescription |
| Company | Sandoz; Watson Labs; Teva Pharms; Mutual Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Quinidine sulfate |
| Drug Label | Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the -isomer of quinine, and its molecular weight is 324.43. dQuinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,... |
| Active Ingredient | Quinidine sulfate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 200mg; 300mg |
| Market Status | Prescription |
| Company | Sandoz; Watson Labs; Teva Pharms; Mutual Pharm |
Adrenergic alpha-Antagonists; Anti-Arrhythmia Agents; Antimalarials; Enzyme Inhibitors; Muscarinic Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Quinidine is indicated in the treatment of recurrent, documented, life-threatening ventricular arrhythmias, such as sustained ventricular tachycardia. Quinidine should not be used to treat ventricular arrhythmias of lesser severity, such as asymptomatic ventricular premature contractions. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2482
Quinidine is indicated in the treatment of symptomatic atrial fibrillation or flutter in patients whose symptoms are not controlled by measures to reduce the rate of ventricular response. /US Product Labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2483
Chronic quinidine treatment is indicated for some patients at high risk for symptomatic atrial fibrillation pr flutter, such as those who have had previous episodes taht are so frequent and poorly tolerated as to outweigh the risk of porphylactic therapy with quinidine. /US Product Labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2483
Intravenous quinidine is indicated in the treatment of life-threatening Plasmodium falciparum malaria. /US Product Labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2483
Skin reactions to quinidine are rare and include morbilliform and scarlatiniformeruptions, urticaria, rash, pruritus, exfoliative dermatitis, eczema, severe exacerbation of psoriasis, lichenoid reactions, flushing, pigmentary abnormalities, photodermatitis (photosensitivity), and contact dermatitis.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1566
... Adverse reactions are not related to plasma concentration and include drug fever cholestatic hepatitis, systemic lupus erythematosus, asthma, anaphylaxis, thrombocytopenia, hemolytic anemia (especially in glutcose-6-phosphate dehydrogenase deficiency), and hypoprothrombinemia. Skin changes range from maculopapular eruption to thrombocytopenic purpura, cutaneous vasculitis, photosensitivity, and bullous lesions.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 511
Drug induced agranulocytosisis a clinical entity characterized by a selective reduction of circulating neutrophils, usually to a level less than 0.2X10+9/l in relation to the administration of the drug. Quinidine is an antiarrhythmic agent widely used on an outpatient basis with some well known hematological side effects. Its midterm administration has been related to a few cases of agranulocytosis. The case of a 60 yr old man with atrial fibrillation is described who presented quinidine induced agranulocytosis of abrupt onset only 3 days after the exposure to the drug, recovering normal levels of neutrophils during the third hospitalization day.
PMID:2117328 Sureda A et al; Acta Haematol 84 (1): 43-4 (1990)
Large doses ...increase temporal dispersion of ventricular refractory periods ...may induce idioventricular impulse generation. Caution ...mandatory in ...treatment of ventricular ectopic rhythms; increased dose after therapeutic failure may increase hazard.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 689
For more Drug Warnings (Complete) data for QUINIDINE (34 total), please visit the HSDB record page.
For the treatment of ventricular pre-excitation and cardiac dysrhythmias
FDA Label
Quinidine, a hydantoin anticonvulsant, is used alone or with phenobarbital or other anticonvulsants to manage tonic-clonic seizures, psychomotor seizures, neuropathic pain syndromes including diabetic neuropathy, digitalis-induced cardiac arrhythmias, and cardiac arrhythmias associated with QT-interval prolongation.
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
Cytochrome P-450 CYP2D6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2D6. (See all compounds classified as Cytochrome P-450 CYP2D6 Inhibitors.)
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
Adrenergic alpha-Antagonists
Drugs that bind to but do not activate alpha-adrenergic receptors thereby blocking the actions of endogenous or exogenous adrenergic agonists. Adrenergic alpha-antagonists are used in the treatment of hypertension, vasospasm, peripheral vascular disease, shock, and pheochromocytoma. (See all compounds classified as Adrenergic alpha-Antagonists.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
C - Cardiovascular system
C01 - Cardiac therapy
C01B - Antiarrhythmics, class i and iii
C01BA - Antiarrhythmics, class ia
C01BA01 - Quinidine
Route of Elimination
When the urine pH is less than 7, about 20% of administered quinidine appears unchanged in the urine, but this fraction drops to as little as 5% when the urine is more alkaline.
Volume of Distribution
2 to 3 L/kg
0.5 L/kg [congestive heart failure]
3 to 5 L/kg [cirrhosis of the liver]
Clearance
3 5 mL/min/kg [adults]
The volume of distribution of quinidine is 2 to 3 L/kg in healthy young adults, but this may be reduced to as little as 0.5 L/kg in patients with congestive heart failure, or increased to 3 or 5 L/kg in patients with cirrhosis of the liver. At concentrations of 2 to 5 mg/L (6.5 to 16.2 umol/L), the fraction of quinidine bound to plasma proteins (mainly to (alpha)1-acid glycoprotein and to albumin) is 80 to 88% in adults and older children, but it is lower in pregnant women, and in infants and neonates it may be as low as 50 to 70%. Because (alpha)1-glycoprotein levels are increased in response to stress, serum levels of total quinidine may be greatly increased in settings such as acute myocardial infarction, even though the serum content of unbound (active) drug may remain normal. Protein binding is also increased in chronic renal failure, but binding abruptly descends toward or below normal when heparin is administered for hemodialysis.
Medical Economics Co; Physicians Desk Reference 56th ed p. 2933 (2002)
...Essentially completely absorbed after oral admin; max effects occur within 1-3 hr, and persist for 6-8 more hr. Large fluctuations in plasma concentration... if repeated doses are given at this interval. ...IM admin... gluconate yields peak effects in 30-90 min.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 291
...All administered compound is excreted by kidney, and about 10-50% appears in urine as unchanged quinidine, within 24 hr.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 692
The bioavailability of quinidine is 70 to 80% after oral use but varies between individuals and preparations. The sulfate salt is rapidly absorbed in 60 to 90 minutes. Polygalacturonate salts produce peak quinidine concentrations in 5 to 6 hours; gastrointestinal absorption of gluconate salts is intermediate (peak 3-4 hours).
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 510
For more Absorption, Distribution and Excretion (Complete) data for QUINIDINE (14 total), please visit the HSDB record page.
Lactate conjugates of quinidine and its 3-hydroxy metabolite were detected in overdose suicide patient.
Leferink et al; J Anal Toxicol 1 (2): 62-5 (1977)
Quinidine is metabolized in the liver, principally via hydroxylation to 3-hydroxyquinidine and 2-quinidinone. Some metabolites have antiarrhythmic activity. Approximately 10-50% of a dose is excreted in urine (probably by glomerular filtration) as unchanged drug within 24 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1571
Quinidine metabolites include 3-hydroxyquinidine N-oxide, 2'-oxoquinidinone, desmethylquinidine, and quinidine N-oxide. While metabolism is highly variable between individuals, at least in cases of quinidine-induced torsade de pointes, the metabolites do not appear to contribute to the formation of dysrhythmias.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 510
Quinidine undergoes extensive hepatic oxidative metabolism... One metabolite, 3-hydroxyquinidine, is nearly as potent as quinidine in blocking cardiac sodium channels or prolonging action potentials.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 966
Most quindiidne is eliminated hepatically via the action of cytochrome P450 IIIA.
Medical Economics Co; Physicians Desk Reference 56th ed p. 2933 (2002)
Quinidine has known human metabolites that include 3-Hydroxyquinidine and Quinidine-N-oxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
6-8 hours
Quinidine generally has a plasma half-life of 6-8 hr in healthy inividuals, but half-life may range from 3-16 hr or longer. In one study in patients with Plasmodium falciparum malaria, the elimination half-life of the drug averaged 12.8 hr (range: 6.6-24.8 hr).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1571
The usual plasma half-life of approximately 7 hours after intravenous administration is increased in the presence of chronic liver disease.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 510
Quinidine acts on sodium channels on the neuronal cell membrane, limiting the spread of seizure activity and reducing seizure propagation. The antiarrhythmic actions are mediated through effects on sodium channels in Purkinje fibers. Quinidine may also act on the slow inward calcium current (ICa), the rapid (IKr) and slow (IKs) components of the delayed potassium rectifier current, the inward potassium rectifier current (IKI), the ATP-sensitive potassium channel (IKATP) and Ito.
The exact mechanism of antiarrhythmic action of quinidine has not been determined conclusively, but the drug is considered a class I (membrane stabilizing) antiarrhythmic agent. Like other class I antiarrhythmic agents, quinidine is believed to combine with fast sodium channels in their inactive state and thereby inhibit recovery after repolarization in a time- and voltage-dependent manner, which is associated with subsequent dissociation of the drug from the sodium channels. Quinidine exhibits electrophysiologic effects characteristic of class IA antiarrhythmic agents. The electrophysiologic characteristics of the subgroups of class I antiarrhythmic agents may be related to quantitative differences in their rates of attachment to and dissociation from transmembrane sodium channels, with class IA agents exhibiting intermediate rates of attachment and dissociation.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1570
Like lidocaine and procainamide, quinidine suppresses automaticity in the His-Purkinje system. In usual doses, quinidine may decrease the automaticity of ectopic pacemakers, but the extent of this effect also depends upon the anticholinergic effect of the drug on the sinoatrial node, atria, and atrioventricular node. Extremely high concentrations of quinidine may increase myocardial automaticity. The drug decreases conduction velocity in the atria, ventricles, and His-Purkinje system, and may decrease or cause no change in conduction velocity through the AV node. Quinidine probably suppresses atrial fibrillation or flutter by prolonging the effective refractory period and increasing the action potential duration in atrial and ventricular muscle and in the His-Purkinje system. Because prolongation of the effective refractory period is greater than the increase in the duration of the action potential, the cardiac tissue remains refractory even after restoration of the resting membrane potential. Quinidine shortens the effective refractory period of the atrioventricular node, and the anticholinergic action of the drug may also increase the conductivity of the atrioventricular node. The effects of quinidine on refractoriness and the action potential duration of atrial fibers may be modified by the anticholinergic effects of the drug. Quinidine decreases cardiac excitability, both in diastole and in the relative refractory period, by increasing the threshold potential for electrical excitation. At therapeutic plasma concentrations, quinidine causes prolongation of the QRS complex and QT interval.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1570
Quinidine also exhibits some antipyretic and oxytocic properties. Quinidine has a very weak curare-like action on the myoneural junction and also causes depression of skeletal muscle action potential.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1570
Intravenous quinidine depresses contractility and decreases systemic vascular resistance primarily by alpha-adrenergic receptor blockade. High blood levels of quinidine increase left ventricular end-diastolic pressure through its negative inotropic effect. Cardiovascular collapse has resulted from depression of contractility.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 511
Quindine primarily kills the schizont parasite at the asexual intra-erythrocytic cycle stage of the Plasmodium falciparum malaria protozoan parasite. Quinidine also kills the gametocyte parasite stages of Plasmodium malariae, Plasmodium vivax, and Plasmodium ovale.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 2483

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
23
PharmaCompass offers a list of Quinidine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Quinidine manufacturer or Quinidine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Quinidine manufacturer or Quinidine supplier.
PharmaCompass also assists you with knowing the Quinidine API Price utilized in the formulation of products. Quinidine API Price is not always fixed or binding as the Quinidine Price is obtained through a variety of data sources. The Quinidine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Quinidine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Quinidine, including repackagers and relabelers. The FDA regulates Quinidine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Quinidine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Quinidine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Quinidine supplier is an individual or a company that provides Quinidine active pharmaceutical ingredient (API) or Quinidine finished formulations upon request. The Quinidine suppliers may include Quinidine API manufacturers, exporters, distributors and traders.
click here to find a list of Quinidine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Quinidine DMF (Drug Master File) is a document detailing the whole manufacturing process of Quinidine active pharmaceutical ingredient (API) in detail. Different forms of Quinidine DMFs exist exist since differing nations have different regulations, such as Quinidine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Quinidine DMF submitted to regulatory agencies in the US is known as a USDMF. Quinidine USDMF includes data on Quinidine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Quinidine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Quinidine suppliers with USDMF on PharmaCompass.
Quinidine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Quinidine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Quinidine GMP manufacturer or Quinidine GMP API supplier for your needs.
A Quinidine CoA (Certificate of Analysis) is a formal document that attests to Quinidine's compliance with Quinidine specifications and serves as a tool for batch-level quality control.
Quinidine CoA mostly includes findings from lab analyses of a specific batch. For each Quinidine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Quinidine may be tested according to a variety of international standards, such as European Pharmacopoeia (Quinidine EP), Quinidine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Quinidine USP).
