

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
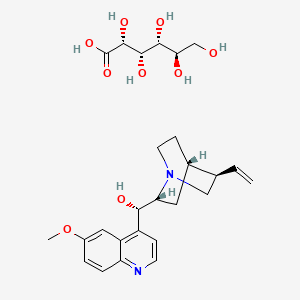

1. Quinact
2. Quinaglute
3. Quinidine Gluconate, (8alpha,9r)-isomer
4. Quinidine Gluconate, (9s)-isomer
1. Duraquin
2. 7054-25-3
3. Gluquinate
4. Dura-tab
5. Quinidine Mono-d-gluconate
6. 6587-33-3
7. Nsc-757297
8. Gluconic Acid Quinidine Salt
9. Quinidine Monogluconate, D-
10. Chebi:27502
11. R6875n380f
12. Quinidine Gluconate Salt
13. Cinchonan-9-ol, 6'-methoxy-, (9s)-, Mono-d-gluconate (salt)
14. D-gluconic Acid, Compd. With (9s)-6'-methoxycinchonan-9-ol (1:1)
15. Quinidine Gluconate [usp]
16. (s)-[(2r,4s,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol;(2r,3s,4r,5r)-2,3,4,5,6-pentahydroxyhexanoic Acid
17. Quinidine D-gluconate
18. Sr-05000001710
19. Quinidine, D-gluconate (salt)
20. Unii-r6875n380f
21. Quinidine Mono-d-gluconate (salt)
22. Einecs 229-517-1
23. Einecs 230-333-9
24. Spectrum_000910
25. Spectrum2_000408
26. Spectrum3_000893
27. Spectrum4_000987
28. Spectrum5_001080
29. Schembl41479
30. Kbiogr_001393
31. Kbioss_001390
32. Divk1c_000228
33. Spectrum1500523
34. Spbio_000336
35. Chembl1200437
36. Quinidine Gluconate [mi]
37. Schembl15541190
38. Hms500l10
39. Hy-b1751f
40. Kbio1_000228
41. Kbio2_001390
42. Kbio2_003958
43. Kbio2_006526
44. Kbio3_001806
45. Ninds_000228
46. Dtxsid801027497
47. Hms1920n18
48. Hms2092e19
49. Pharmakon1600-01500523
50. Quinidine Gluconate [vandf]
51. D-gluconic Acid, Compound With (9s)-6'-methoxycinchonan-9-ol
52. Quinidine Gluconate [mart.]
53. Ccg-38674
54. Nsc757297
55. Quinidine Gluconate [usp-rs]
56. Quinidine Gluconate [who-dd]
57. Ex-6000
58. Nsc 757297
59. Idi1_000228
60. Quinidine Gluconate [orange Book]
61. Quinidine Gluconate [usp Monograph]
62. Cs-0030857
63. Quinidine Gluconate Salt, Analytical Standard
64. 054q253
65. Gluconic Acid, Compd. With Quinidine (1:1), D-
66. Sr-05000001710-1
67. Sr-05000001710-2
68. W-110193
69. Q27094388
70. (9s)-6'-methoxycinchonan-9-ol--d-gluconic Acid (1:1)
71. Cinchonan-9-ol, 6'-methoxy-, (9s)-, Mono-d-gluconate
72. D-gluconic Acid, Compd. With (9s)-6'-methoxycinchonan-9-ol (1:?)
73. Quinidine Gluconate, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 520.6 g/mol |
|---|---|
| Molecular Formula | C26H36N2O9 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 9 |
| Exact Mass | 520.24208073 g/mol |
| Monoisotopic Mass | 520.24208073 g/mol |
| Topological Polar Surface Area | 184 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 627 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
Related Excipient Companies

Excipients by Applications
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
