
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
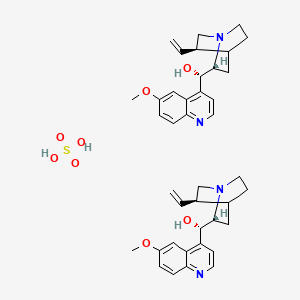

1. Adaquin
2. Apo Quinidine
3. Apo-quinidine
4. Chinidin
5. Quincardine
6. Quinidex
7. Quinidine
8. Quinidine Sulfate
9. Quinora
10. Sulfate, Quinidine
1. Quinidine Sulfate
2. 50-54-4
3. Dtxsid80911600
4. Hms3263i19
5. Ccg-222313
6. Lp01009
7. Eu-0101009
8. Q0010
9. Q 0875
10. Sulfuric Acid--6'-methoxycinchonan-9-ol (1/2)
11. 11034-22-3
| Molecular Weight | 746.9 g/mol |
|---|---|
| Molecular Formula | C40H50N4O8S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Exact Mass | 746.33493574 g/mol |
| Monoisotopic Mass | 746.33493574 g/mol |
| Topological Polar Surface Area | 174 Ų |
| Heavy Atom Count | 53 |
| Formal Charge | 0 |
| Complexity | 538 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Quinidine sulfate |
| Drug Label | Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the -isomer of quinine, and its molecular weight is 324.43. dQuinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,... |
| Active Ingredient | Quinidine sulfate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 200mg; 300mg |
| Market Status | Prescription |
| Company | Sandoz; Watson Labs; Teva Pharms; Mutual Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Quinidine sulfate |
| Drug Label | Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the -isomer of quinine, and its molecular weight is 324.43. dQuinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,... |
| Active Ingredient | Quinidine sulfate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 200mg; 300mg |
| Market Status | Prescription |
| Company | Sandoz; Watson Labs; Teva Pharms; Mutual Pharm |
MEDICATION (VET): OF 6 ANTIARRHYTHMICS TESTED, QUINIDINE BISULFATE GAVE NO PROTECTION AGAINST THE INDUCED ARRHYTHMIA IN DOGS.
FERNANDEZ MZ ET AL; REV COLOMB CIENC QUIM-FARM 3 (3): 65 (1979)
... IS EFFECTIVE FOR SHORT- AND LONG-TERM TREATMENT OF SUPRAVENTRICULAR AND VENTRICULAR ARRHYTHMIAS. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 854
FOR PRACTICAL PURPOSES, QUINIDINE IS ONLY GIVEN ORALLY, ALTHOUGH IT CAN BE ADMINEITHER IM OR IV UNDER SPECIAL CIRCUMSTANCES. THE USUAL ORAL DOSE OF QUINIDINE SULFATE IS 200 TO 300 MG THREE TO FOUR TIMES A DAY. ... FOR PATIENTS WITH PREMATURE ATRIAL OR VENTRICULAR CONTRACTIONS OR MAINTENANCE THERAPY. HIGHER AND/OR MORE FREQUENT DOSES CAN BE USED FOR LIMITED PERIODS FOR TREATMENT OF PAROXYSMAL VENTRICULAR TACHYCARDIA.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 853
Quinidine is used primarily as prophylactic therapy to maintain normal sinus rhythm after conversion of atrial fibrillation and/or flutter by other methods. The drug is also used to prevent the recurrence of paroxysmal atrial fibrillation, paroxysmal atrial tachycardia, paroxysmal atrioventricular junctional rhythm, paroxysmal ventricular tachycardia, and atrial or ventricular premature contractions.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 993
For more Therapeutic Uses (Complete) data for QUINIDINE SULFATE (7 total), please visit the HSDB record page.
OCCASIONALLY PATIENTS TAKING QUINIDINE EXPERIENCE SYNCOPE OR SUDDEN DEATH. ... MAY BE RESULT OF HIGH CONCENTRATIONS OF QUINIDINE IN PLASMA OR RESULT OF COEXISTING DIGITALIS TOXICITY. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 853
INDIVIDUALS WITH THE LONG Q-T SYNDROME OR THOSE WHO RESPOND TO LOW CONCENTRATIONS OF QUINIDINE WITH MARKED LENGTHENING OF THE Q-T INTERVAL APPEAR TO BE PARTICULARLY AT RISK /OF SYNCOPE OR SUDDEN DEATH/ AND SHOULD NOT BE TREATED WITH THIS DRUG. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 853
EXCESSIVE CONCENTRATION OF DRUG IN PLASMA WILL CAUSE ADVERSE EFFECTS IN ANY PATIENT. BECAUSE QUINIDINE HAS LOW THERAPEUTIC RATIO, CONSTANT VIGILANCE IS THUS REQUIRED IN EVERY PATIENT TAKING THIS DRUG. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 853
Quinidine should be used with extreme caution, if at all, in patients with incomplete atrioventricular nodal block, since complete heart block and asystole may result. Im or iv administration of quinidine is especially hazardous in the presence of atrioventricular block, in the absence of atrial activity, and the patients with extensive myocardial injury. Hypokalemia, hypoxia, and disorders of acid base balance must be eliminated as potentiating factors in patients who require large doses of antiarrhythmic agents to control ventricular arrhythmias.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 995
For more Drug Warnings (Complete) data for QUINIDINE SULFATE (24 total), please visit the HSDB record page.
Adrenergic alpha-Antagonists
Drugs that bind to but do not activate alpha-adrenergic receptors thereby blocking the actions of endogenous or exogenous adrenergic agonists. Adrenergic alpha-antagonists are used in the treatment of hypertension, vasospasm, peripheral vascular disease, shock, and pheochromocytoma. (See all compounds classified as Adrenergic alpha-Antagonists.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)
Voltage-Gated Sodium Channel Blockers
A class of drugs that inhibit the activation of VOLTAGE-GATED SODIUM CHANNELS. (See all compounds classified as Voltage-Gated Sodium Channel Blockers.)
Cytochrome P-450 CYP2D6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2D6. (See all compounds classified as Cytochrome P-450 CYP2D6 Inhibitors.)
ABOUT 90% OF QUINIDINE IN PLASMA IS BOUND TO PLASMA PROTEINS (ALPHA/ACID GLYCOPROTEIN AND ALBUMIN) THE DRUG ENTERS ERYTHROCYTES & ... BINDS TO HEMOGLOBIN; AT STEADY STATE, CONCN OF QUINIDINE IN PLASMA & ERYTHROCYTES ARE APPROXIMATELY EQUAL. QUINIDINE ACCUMULATES RAPIDLY IN MOST TISSUES EXCEPT BRAIN, & ... VOL OF DISTRIBUTION IS 2-3 L/KG. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 852
METABOLITES AND SOME OF THE PARENT DRUG (20%) ARE EXCRETED IN URINE; ELIMINATION HALF-TIME IS ABOUT 6 HR. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 852
LIVER METABOLISM & RENAL EXCRETION ARE THE MAIN ROUTES OF ELIMINATION. ENTEROHEPATIC CIRCULATION WOULD NOT SIGNIFICANTLY ALTER ABSORPTION KINETICS AS REFLECTED BY BLOOD CONCENTRATION.
PMID:7264866 KOSKAS J ET AL; J PHARM BELG 36 (3): 193 (1981)
PEAK PLASMA CONCN OF 0.29 UG/ML OF QUINIDINE WERE MEASURED @ 4 HR AFTER ADMIN OF SUSTAINED RELEASE CAPSULE (250 MG QUINIDINE BISULFATE) AND DECLINED STEADILY DURING THE NEXT 8 HR, WHILE AFTER ADMIN OF SUSTAINED RELEASE TABLET (300 MG QUINIDINE SULFATE) THEY WERE FAIRLY EVEN DURING 2-10 HR AFTER DOSING. PLASMA CONCENTRATIONS WERE HIGHER AT LATER TIMES FOR THE CAPSULE THAN FOR THE TABLET. THE BIOAVAILABILITY OF QUINIDINE FROM THE CAPSULES DURING 12 HR WAS 184% COMPARED TO THE TABLET. MEAN QUINIDINE PLASMA CONCN WERE SIGNIFICANTLY GREATER @ 3, 4, 6, 8, & 10 HR AFTER ADMIN OF THE CAPSULE THAN AFTER THE TABLET.
CHASSEAUD LF ET AL; PHARM IND 38 (5): 488 (1976)
For more Absorption, Distribution and Excretion (Complete) data for QUINIDINE SULFATE (24 total), please visit the HSDB record page.
QUINIDINE YIELDS 2'-HYDROXYQUINIDINE AS METABOLITE IN MAN. /QUINIDINE; FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. Q-3
MOST URINARY METABOLITES ARE HYDROXYLATED AT ONLY ONE SITE, EITHER ON THE QUINOLINE RING OR ON THE QUINUCLIDINE RING; SMALL AMOUNTS OF DIHYDROXY COMPOUNDS ARE ALSO FOUND. THE FRACTION OF A DOSE OF QUINIDINE THAT IS METABOLIZED & THE METABOLIC PATHWAY APPEAR TO VARY CONSIDERABLY FROM PATIENT TO PATIENT.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 852
Quinidine is metabolized in the liver, principally via hydroxylation to 3-hydroxyquinidine and 2-quinidinone. The metabolites may be pharmacologically active. Approximately 10-50% of a dose is excreted in urine (probably by glomerular filtration) as unchanged drug within 24 hr. /Quinidine/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 993
THE ELIMINATION HALF-LIFE OF QUINIDINE RANGES FROM 4 TO 10 HR IN HEALTHY PERSONS, WITH USUAL MEAN VALUE OF 6 TO 7 HR. HALF-LIFE IS SIGNIFICANTLY PROLONGED IN ELDERLY PERSONS, EVEN WHEN THEY ARE APPARENTLY HEALTHY. /QUINIDINE/
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 299
... EXCRETED IN URINE; ELIMINATION HALF-TIME IS ABOUT 6 HR. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 852
Quinidine generally has a plasma half-life of 6-8 hr in healthy individuals, but half-life may range from 3-16 hr or longer. In one study in patients with Plasmodium falciparum malaria, the elimination half-life of the drug averaged 12.8 hr (range: 6.6-24.8 hr). /Quinidine/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 93. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1993 (Plus Supplements, 1993)., p. 993
IN EXPERIMENTAL ANIMALS, QUINIDINE HAS VERY SIGNIFICANT ATROPINE LIKE ACTION, BLOCKING EFFECTS OF VAGAL STIMULATION OR OF ACETYLCHOLINE. ... ALSO HAS ALPHA-ADRENERGIC BLOCKING PROPERTIES. THIS CAN CAUSE VASODILATATION &, VIA BARORECEPTORS, ACTIVE SYMPATHETIC EFFERENT ACTIVITY. TOGETHER, CHOLINERGIC BLOCKAGE & INCR BETA-ADRENERGIC ACTIVITY CAUSED BY QUINIDINE CAN INCR SINUS RATE & ENHANCE ATRIOVENTRICULAR NODAL CONDUCTION. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 851
CAN CAUSE SEVERE DEPRESSION OF SINUS NODE IN PATIENTS WITH THE SICK SINUS SYNDROME ... QUINIDINE CAN INCR SINUS RATE BY CHOLINERGIC BLOCKADE OR BY REFLEXLY INCR SYMPATHETIC ACTIVITY. ... THERAPEUTIC CONCN OF QUINIDINE ... DECR FIRING RATE OF CARDIAC PURKINJE FIBERS BY DIRECT ACTION ... DECR SLOPE OF PHASE 4 DEPOLARIZATION AND SHIFTS THRESHOLD VOLTAGE TOWARD ZERO. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 850
... INCR DIASTOLIC ELECTRICAL CURRENT THRESHOLD IN ATRIAL & VENTRICULAR MUSCLE & IN PURKINJE FIBERS ... ALSO INCR FIBRILLATION THRESHOLD IN ATRIA & VENTRICLES. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 850
REENTRANT ARRYTHMIAS ARE ABOLISHED BY /QUINIDINE/. THEIR EFFECT ON EFFECTIVE REFRACTORY PERIOD, RESPONSIVENESS, & CONDUCTION. FOR EXAMPLE, WHEN VENTRICULAR PREMATURE DEPOLARIZATIONS ARE CAUSED BY REENTRY IN LOOPS OF PURKINJE FIBERS, ONE WAY BLOCK CAN BE CONVERTED TO TWO WAY BLOCK, THUS MAKING REENTRY IMPOSSIBLE. /QUINIDINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 850
For more Mechanism of Action (Complete) data for QUINIDINE SULFATE (9 total), please visit the HSDB record page.

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Application : Emulsifying Agents
Excipient Details : HDK N20 Pharma is used as a pharmaceutical emulsifying agent in tablets, capsules, syrups, and solutions.
Dosage Form : Cream / Lotion / Ointment, Tablet
Grade : Oral
Category : Emulsifying Agents, Lubricants & Glidants, Thickeners and Stabilizers
Application : Emulsifying Agents, Lubricants & Glidants, Thickeners and Stabilizers
Application : Emulsifying Agents, Lubricants & Glidants
Excipient Details : Glidant; Emulsion Stabilizer; Anti-caking Agent.
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
A Quinidine Sulfate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Quinidine Sulfate, including repackagers and relabelers. The FDA regulates Quinidine Sulfate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Quinidine Sulfate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Quinidine Sulfate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Quinidine Sulfate supplier is an individual or a company that provides Quinidine Sulfate active pharmaceutical ingredient (API) or Quinidine Sulfate finished formulations upon request. The Quinidine Sulfate suppliers may include Quinidine Sulfate API manufacturers, exporters, distributors and traders.
click here to find a list of Quinidine Sulfate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Quinidine Sulfate DMF (Drug Master File) is a document detailing the whole manufacturing process of Quinidine Sulfate active pharmaceutical ingredient (API) in detail. Different forms of Quinidine Sulfate DMFs exist exist since differing nations have different regulations, such as Quinidine Sulfate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Quinidine Sulfate DMF submitted to regulatory agencies in the US is known as a USDMF. Quinidine Sulfate USDMF includes data on Quinidine Sulfate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Quinidine Sulfate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Quinidine Sulfate suppliers with USDMF on PharmaCompass.
A Quinidine Sulfate CEP of the European Pharmacopoeia monograph is often referred to as a Quinidine Sulfate Certificate of Suitability (COS). The purpose of a Quinidine Sulfate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Quinidine Sulfate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Quinidine Sulfate to their clients by showing that a Quinidine Sulfate CEP has been issued for it. The manufacturer submits a Quinidine Sulfate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Quinidine Sulfate CEP holder for the record. Additionally, the data presented in the Quinidine Sulfate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Quinidine Sulfate DMF.
A Quinidine Sulfate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Quinidine Sulfate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Quinidine Sulfate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Quinidine Sulfate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Quinidine Sulfate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Quinidine Sulfate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Quinidine Sulfate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Quinidine Sulfate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Quinidine Sulfate suppliers with NDC on PharmaCompass.
Quinidine Sulfate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Quinidine Sulfate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Quinidine Sulfate GMP manufacturer or Quinidine Sulfate GMP API supplier for your needs.
A Quinidine Sulfate CoA (Certificate of Analysis) is a formal document that attests to Quinidine Sulfate's compliance with Quinidine Sulfate specifications and serves as a tool for batch-level quality control.
Quinidine Sulfate CoA mostly includes findings from lab analyses of a specific batch. For each Quinidine Sulfate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Quinidine Sulfate may be tested according to a variety of international standards, such as European Pharmacopoeia (Quinidine Sulfate EP), Quinidine Sulfate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Quinidine Sulfate USP).


LOOKING FOR A SUPPLIER?