


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
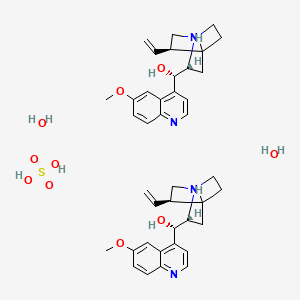

1. Quinidex (tn)
2. Quinidine Sulfate (usp)
3. Dtxsid80975964
4. Quinidine Sulfate Hydrate (jp17)
5. Akos015895974
6. D02272
7. Sulfuric Acid--6'-methoxycinchonan-9-ol--water (1/2/2)
8. (s)-[(2r,5r)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol;sulfuric Acid;dihydrate
9. 60553-86-8
| Molecular Weight | 782.9 g/mol |
|---|---|
| Molecular Formula | C40H54N4O10S |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 8 |
| Exact Mass | 782.35606511 g/mol |
| Monoisotopic Mass | 782.35606511 g/mol |
| Topological Polar Surface Area | 176 Ų |
| Heavy Atom Count | 55 |
| Formal Charge | 0 |
| Complexity | 538 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
| 1 of 2 | |
|---|---|
| Drug Name | Quinidine sulfate |
| Drug Label | Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the -isomer of quinine, and its molecular weight is 324.43. dQuinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,... |
| Active Ingredient | Quinidine sulfate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 200mg; 300mg |
| Market Status | Prescription |
| Company | Sandoz; Watson Labs; Teva Pharms; Mutual Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Quinidine sulfate |
| Drug Label | Quinidine is an antimalarial schizonticide and an antiarrhythmic agent with class 1A activity; it is the -isomer of quinine, and its molecular weight is 324.43. dQuinidine sulfate is the sulfate salt of quinidine; its chemical name is cinchonan-9-ol,... |
| Active Ingredient | Quinidine sulfate |
| Dosage Form | Tablet, extended release; Tablet |
| Route | Oral |
| Strength | 200mg; 300mg |
| Market Status | Prescription |
| Company | Sandoz; Watson Labs; Teva Pharms; Mutual Pharm |


