
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 43285, Rs
2. Dihydrochloride, Ranolazine
3. Hcl, Ranolazine
4. Hydrochloride, Ranolazine
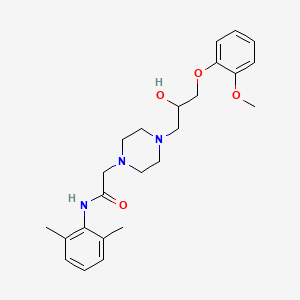
5. N-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-1-piperazineacetamide
6. Ranexa
7. Ranolazine Dihydrochloride
8. Ranolazine Hcl
9. Ranolazine Hydrochloride
10. Renolazine
11. Rs 43285
12. Rs 43285 193
13. Rs 43285-193
14. Rs 43285193
15. Rs-43285
16. Rs43285
1. 95635-55-5
2. Ranexa
3. N-(2,6-dimethylphenyl)-2-(4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)piperazin-1-yl)acetamide
4. Cvt-303
5. Ran D
6. Ran4
7. N-(2,6-dimethylphenyl)-2-[4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl]acetamide
8. 142387-99-3
9. Rs-43285-003
10. Ranolazine (ranexa)
11. N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl}acetamide
12. Chebi:87690
13. Nsc-759100
14. A6iez5m406
15. Cvt 303
16. Ncgc00015897-02
17. Rs 43285-003
18. Dsstox_cid_25196
19. Dsstox_rid_80743
20. Dsstox_gsid_45196
21. (-)-ranolazine
22. Latixa
23. 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-
24. N-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-1-piperazineacetamide
25. N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]-1-piperazineacetamide
26. (+-)-ranolazine
27. Cas-95635-55-5
28. Ranexa (tn)
29. Ranolazine [usan]
30. Sr-01000076216
31. Ranolazine (usan/inn)
32. Unii-a6iez5m406
33. Ranolazine [usan:inn:ban]
34. Hsdb 7924
35. Keg-1295
36. Ranolazine- Bio-x
37. Aspruzyo
38. (+/-)-ranolazine
39. Ranolazine [mi]
40. Ranolazine [inn]
41. Ranolazine [vandf]
42. Ranolazine [mart.]
43. Chembl1404
44. Ranolazine [usp-rs]
45. Ranolazine [who-dd]
46. Lopac0_001062
47. Bspbio_002276
48. Mls002154149
49. Ranolazine [ema Epar]
50. Schembl124665
51. Spectrum1505366
52. Gtpl7291
53. Dtxsid3045196
54. Ranolazine [orange Book]
55. Hms1922f16
56. Hms2090l09
57. Hms2093d21
58. Hms2098k06
59. Hms2230c19
60. Hms3369i08
61. Hms3655m12
62. Hms3715k06
63. Hms3884i10
64. Pharmakon1600-01505366
65. Act06286
66. Amy14177
67. Bcp04190
68. Hy-b0280
69. Tox21_110258
70. Bdbm50173335
71. Mfcd00864690
72. Nsc759100
73. Nsc782305
74. S1799
75. Akos015889500
76. Tox21_110258_1
77. Ac-1673
78. Bcp9000558
79. Ccg-205139
80. Db00243
81. Ks-1244
82. Nsc 759100
83. Nsc-782305
84. Pb21724
85. Rs43285
86. Sb13209
87. Sdccgsbi-0051032.p003
88. Ncgc00015897-03
89. Ncgc00015897-04
90. Ncgc00015897-05
91. Ncgc00015897-06
92. Ncgc00015897-08
93. Ncgc00015897-22
94. Ncgc00095177-01
95. Ncgc00095177-02
96. Ncgc00095177-03
97. 110445-25-5
98. Bd164322
99. Smr000857382
100. Bcp0726000090
101. Sbi-0051032.p002
102. Ft-0601594
103. Ft-0674327
104. Sw197620-4
105. D05700
106. Ab00698532-11
107. Ab00698532-13
108. Ab00698532-14
109. Ab00698532-15
110. Ab00698532_16
111. Ab00698532_17
112. Ab00698532_18
113. 635r555
114. Q907104
115. Sr-01000076216-5
116. Sr-01000076216-8
117. Brd-a97674275-001-01-9
118. Brd-a97674275-001-04-3
119. Z68563450
120. (+/-)-4-(2-hydroxy-3-(o-methoxyphenoxy)propyl)-1-piperazineaceto-2',6'-xylidide
121. (+-)-n-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-1-piperazineacetamide
122. (+/-)-1-(3-(2-methoxyphenoxy)-2-hydroxypropyl)-4-(n-(2,6-dimethylphenyl)carbamoylmethyl)piperazine
123. (+/-)-n-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-1-piperazineacetamide
124. 1-[3-(2-methoxyphenoxy)-2-hydroxypropyl]-4-[(2,6-dimethylphenyl)aminocarbonylmethyl]piperazine
125. 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-, (+-)-
126. 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-(2-hydroxy-3-(2-methoxyphenoxy)propyl)-, (+/-)
127. 1080496-58-7
128. N-(2,6-dimethyl-phenyl)-2-{4-[2-hydroxy-3-(2-methoxy-phenoxy)-propyl]-piperazin-1-yl}-acetamide
129. N-(2,6-dimethylphenyl)-2-(4-((2rs)-2-hydroxy-3-(2-methoxyphenoxy)propyl)piperazin-1-yl)acetamide
130. N-(2,6-dimethylphenyl)-2-(4-(2-hydroxy-3-(2-methoxy-phenoxy)propyl)piperazin-1-yl)acetamide
131. N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)-propyl]piperazin-1-yl}-acetamide
132. N-(2,6-dimethylphenyl)-2-{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl]piperazin-1-yl}ethanimidic Acid
| Molecular Weight | 427.5 g/mol |
|---|---|
| Molecular Formula | C24H33N3O4 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 427.24710654 g/mol |
| Monoisotopic Mass | 427.24710654 g/mol |
| Topological Polar Surface Area | 74.3 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 531 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Ranexa |
| PubMed Health | Ranolazine (By mouth) |
| Drug Classes | Antianginal |
| Drug Label | Ranexa (ranolazine) is available as a film-coated, non-scored, extended-release tablet for oral administration.Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)pro... |
| Active Ingredient | Ranolazine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Gilead |
| 2 of 4 | |
|---|---|
| Drug Name | Ranolazine |
| PubMed Health | Ranolazine (By mouth) |
| Drug Classes | Antianginal |
| Drug Label | Ranexa (ranolazine) is available as a film-coated, non-scored, extended-release tablet for oral administration.Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)pro... |
| Active Ingredient | Ranolazine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Lupin |
| 3 of 4 | |
|---|---|
| Drug Name | Ranexa |
| PubMed Health | Ranolazine (By mouth) |
| Drug Classes | Antianginal |
| Drug Label | Ranexa (ranolazine) is available as a film-coated, non-scored, extended-release tablet for oral administration.Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)pro... |
| Active Ingredient | Ranolazine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Gilead |
| 4 of 4 | |
|---|---|
| Drug Name | Ranolazine |
| PubMed Health | Ranolazine (By mouth) |
| Drug Classes | Antianginal |
| Drug Label | Ranexa (ranolazine) is available as a film-coated, non-scored, extended-release tablet for oral administration.Ranolazine is a racemic mixture, chemically described as 1-piperazineacetamide, N-(2,6-dimethylphenyl)-4-[2-hydroxy-3-(2-methoxyphenoxy)pro... |
| Active Ingredient | Ranolazine |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 500mg; 1gm |
| Market Status | Prescription |
| Company | Lupin |
Enzyme Inhibitors; Angina Pectoris/drug therapy
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Ranolazine is indicated for the treatment of chronic angina. Ranolazine may be used with beta-blockers, nitrates, calcium channel blockers, anti-platelet therapy, lipid-lowering therapy, ACE inhibitors, and angiotensin receptor blockers. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
Ranolazine is contraindicated in patients: taking strong inhibitors of CYP3A; taking inducers of CYP3A; with clinically significant hepatic impairment.
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
Ranolazine has been shown to prolong the QT interval corrected for rate (QTc) in a dose-related manner. Although the clinical importance of QTc interval prolongation associated with ranolazine is not known, other drugs with this potential have been associated with torsades de pointes-type arrhythmias and sudden death. The mean effect on QTc interval with repeated dosing of ranolazine 1 g twice daily, at time of maximum plasma concentration (Tmax), is about 6 msec; however, in 5% of the population the prolongation of QTc interval is 15 msec. Age, weight, gender, race, heart rate, NYHA class I to IV CHF, and diabetes have no substantial effect on the relationship between ranolazine plasma concentrations and increases in QTc interval. The relationship between ranolazine concentrations and QTc remains linear over a concentration range up to fourfold greater than the concentrations produced by a ranolazine dosage of 1 g twice daily, and is not affected by changes in heart rate. The manufacturer states that ranolazine dosages exceeding 1 g twice daily should not be used.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1733
The effects of ranolazine in patients with preexisting QT interval prolongation or receiving concomitant therapy with drugs that are known to prolong the QT interval have not been established. Because of possible additive effects on the QT interval, the manufacturer states that use of ranolazine should be avoided in patients with known QT interval prolongation (including congenital long QT syndrome and uncorrected hypokalemia), known history of ventricular tachycardia, and in patients receiving drugs that prolong the QTc interval (eg, class Ia (eg, quinidine) or III (eg, dofetilide, sotalol) antiarrhythmic agents, antipsychotic agents eg, thioridazine, ziprasidone).
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1733
Because the QTc-prolonging effect is increased approximately threefold in patients with hepatic dysfunction, ranolazine is contraindicated in patients with mild, moderate, or severe hepatic impairment.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1733
For more Drug Warnings (Complete) data for Ranolazine (16 total), please visit the HSDB record page.
Ranolazine is indicated for the treatment of chronic angina. It can be used alone or in conjunction with nitrates, beta-blockers, angiotensin receptor blockers, anti-platelet drugs, calcium channel blockers, lipid-lowering drugs, and ACE inhibitors. Ranolazine has also been used off-label for the treatment of certain arrhythmias, including ventricular tachycardia, however, this use is not strongly supported by scientific evidence. Ranolazine has also been studied for the treatment of acute coronary syndrome, microvascular coronary dysfunction, arrhythmia, and glycemic control, which are not yet approved indications.
FDA Label
Ranexa is indicated as add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled or intolerant to first-line anti-anginal therapies (such as beta-blockers and / or calcium antagonists).
Ranolazine exerts both antianginal and ischemic effects independent from lowering heart rate or blood pressure. It blocks IKr, the rapid portion of the delayed rectifier potassium current, and prolongs the QTc interval in a dose-dependent fashion. The Ikr is important for cardiac repolarization. Ranolazine exerts its therapeutic effects without negative chronotropic, dromotropic, or inotropic actions neither at rest, nor during exercise.
Sodium Channel Blockers
A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. (See all compounds classified as Sodium Channel Blockers.)
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
C01EB18
C - Cardiovascular system
C01 - Cardiac therapy
C01E - Other cardiac preparations
C01EB - Other cardiac preparations
C01EB18 - Ranolazine
Absorption
The time to reach peak serum concentration is quite variable but has been observed to be in the range of 2-6 hours, with steady-state within 3 days. The FDA indicates a Tmax of 3-5 hours. The average steady-state Cmax is about 2600 ng/mL. Absorption of ranolazine is not significantly affected by food consumption. The bioavailability of ranolazine taken in the tablet form compared to that from a solution of ranolazine is about 76%.
Route of Elimination
From the administered dose, about 3/4 of the dose is excreted renally, while 1/4 of the dose is excreted in the feces. An estimated 5% of an ingested dose is excreted as unchanged drug.
Volume of Distribution
The mean apparent volume of distribution of ranolazine is reported to be 53.2 L and the average steady-state volume of distribution is estimated to range from 85 to 180 L.
Clearance
The reported clearance rate of orally administered ranolazine is of 45 L/h when administered at a dose of 500 mg twice daily. The clearance rate of ranolazine is dose-dependent and renal impairment can increase ranolazine serum concentration by 40-50%.
Ranolazine is extensively metabolized in the gut and liver and its absorption is highly variable. For example, at a dose of 1000 mg twice daily, the mean steady-state Cmax was 2600 ng/mL with 95% confidence limits of 400 and 6100 ng/mL. The pharmacokinetics of the (+) R- and (-) S-enantiomers of ranolazine are similar in healthy volunteers. ... Steady state is generally achieved within 3 days of twice-daily dosing with ranolazine. At steady state over the dose range of 500 to 1000 mg twice daily, Cmax and AUC0-t increase slightly more than proportionally to dose, 2.2- and 2.4-fold, respectively. With twice-daily dosing, the trough:peak ratio of the ranolazine plasma concentration is 0.3 to 0.6. The pharmacokinetics of ranolazine is unaffected by age, gender, or food.
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
After oral administration of ranolazine, peak plasma concentrations of ranolazine are reached between 2 and 5 hours. After oral administration of (14)C-ranolazine as a solution, 73% of the dose is systemically available as ranolazine or metabolites. The bioavailability of ranolazine from ranolazine tablets relative to that from a solution of ranolazine is 76%. Because ranolazine is a substrate of P-gp, inhibitors of P-gp may increase the absorption of ranolazine.
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
Food (high-fat breakfast) has no important effect on the Cmax and AUC of ranolazine. Therefore, ranolazine may be taken without regard to meals. Over the concentration range of 0.25 to 10 ug/mL, ranolazine is approximately 62% bound to human plasma proteins.
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
It is not known whether ranolazine is distributed into milk.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1733
For more Absorption, Distribution and Excretion (Complete) data for Ranolazine (7 total), please visit the HSDB record page.
Ranolazine is rapidly heavily metabolized in the liver an gastrointestinal tract through the activity of the CYP3A4 enzyme with minor contributions from CYP2D6. More than 40 ranolazine metabolites have been found in plasma and more than 100 metabolites have been identified in the urine. Ranolazine and some of its metabolites are known to weakly inhibit CYP3A4. However, the activity of the metabolites of ranolazine has not been fully elucidated.
Ranolazine is extensively metabolized in the intestine and liver by the cytochrome P-450 (CYP) isoenzyme system, mainly by CYP3A and, to a lesser extent, CYP2D6. In vitro studies indicate that ranolazine also is a p-glycoprotein substrate. At least 4 metabolites of ranolazine have been identified. The pharmacologic activity of these metabolites has not been fully established.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1734
Ranolazine is metabolized rapidly and extensively in the liver and intestine ... The pharmacologic activity of the metabolites has not been well characterized. After dosing to steady state with 500 mg to 1500 mg twice daily, the four most abundant metabolites in plasma have AUC values ranging from about 5 to 33% that of ranolazine...
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
The apparent terminal half-life of ranolazine is 7 hours.
... Elimination half-life of ranolazine is 1.4-1.9 hours but is apparently prolonged, on average, to 7 hours for the ER formulation as a result of extended absorption (flip-flop kinetics). ...
PMID:16640453 Jerling M; Clin Pharmacokinet 45 (5): 469-91(2006)
... The four most abundant metabolites in plasma ... display apparent half-lives ranging from 6 to 22 hours.
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
Myocardial ischemia exerts effects on adenosine triphosphate flux, leading to a decrease in the energy available for contraction and relaxation of the heart muscle. Electrolyte balance of sodium and potassium is necessary for maintaining normal cardiac contraction and relaxation. Disruption of adequate sodium and potassium electrolyte balance leads to excessively high concentrations of sodium and calcium, which likely interferes with oxygen supply to the heart muscle. This imbalance eventually leads to angina symptoms of chest pain or pressure, nausea, and dizziness, among others. The mechanism of action for ranolazine is not fully understood. At therapeutic concentrations, it can inhibit the cardiac late sodium 205 current (INa), which may affect the electrolyte balance in the myocardium, relieving angina symptoms. The clinical significance this inhibition in the treatment of angina symptoms is not yet confirmed. Ranolazine inhibits sodium and potassium ion channel currents. It has been shown to exert weak activity on L-type calcium channels making it a weak direct vasodilator and exerts minimal direct effects on atrioventricular nodal conduction. Some additional mechanisms have been elucidated. Ranolazine exerts antagonistic activity towards the alpha 1 and beta 1 adrenergic receptors and inhibition of fatty acid oxidation.
Ranolazine, a piperazine derivative, is an antianginal agent. Although the exact mechanism of antianginal activity of ranolazine has not been fully elucidated, results of early studies suggested that ranolazine shifted adenosine triphosphate (ATP) production away from fatty acid oxidation (ie, partial inhibition of fatty acid oxidation) in favor of more oxygen-efficient glucose oxidation, especially when free fatty acid concentrations were elevated (eg, during ischemia), leading to reduced oxygen demand and symptoms of ischemia without affecting cardiac work. However, these pharmacologic effects generally were observed at concentrations exceeding therapeutic plasma concentrations in clinical studies.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1734
Recent data suggest that ranolazine may exert its antianginal and anti-ischemic effects through concentration-, voltage-, and frequency-dependent inhibition of the late (ie, sustained, persistent) sodium current and other cardiac ion channels and transporters. The late sodium current is created by inactivation of the sodium channel protein. However, angina (ie, ischemia, hypoxia) impairs sodium channel inactivation and increases the amount of sodium in cardiac cells, which facilitates calcium overload via the sodium-calcium exchange pump. Increased intracellular calcium may result in myocyte hyperexcitability and electrical instability, impaired diastolic relaxation, reduced coronary artery perfusion, impaired myocardial oxygen supply, increased oxygen demand, and ventricular dysfunction. Thus, ranolazine may decrease the magnitude of the late sodium current resulting in a net reduction in intracellular sodium concentrations, reversal of calcium overload, restoration of ventricular pump function, and prevention of ischemia-induced arrhythmias. Unlike other antianginal agents, the antianginal effects of ranolazine are not dependent upon reductions in heart rate or blood pressure.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 1734
The QT prolongation effect of ranolazine on the surface electrocardiogram is the result of inhibition of IKr, which prolongs the ventricular action potential.
US Natl Inst Health; DailyMed. Current Medication Information for RANEXA (ranolazine) tablet, film coated, extended release (March 2010). Available from, as of July 11, 2011: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=739d7705-907b-4408-8490-2052514a1a50
/The authors/ investigated changes in Na(+) currents (I(Na)) in permanent (or chronic) atrial fibrillation (AF) and the effects of I(Na) inhibition using ranolazine (Ran) on arrhythmias and contractility in human atrial myocardium. Electrical remodeling during AF is typically associated with alterations in Ca(2+) and K(+) currents. It remains unclear whether I(Na) is also altered. Right atrial appendages from patients with AF (n = 23) and in sinus rhythm (SR) (n = 79) were studied. Patch-clamp experiments in isolated atrial myocytes showed significantly reduced peak I(Na) density ( approximately 16%) in AF compared with SR, which was accompanied by a 26% lower expression of Nav1.5 (p < 0.05). In contrast, late I(Na) was significantly increased in myocytes from AF atria by approximately 26%. Ran (10 mumol/L) decreased late I(Na) by approximately 60% (p < 0.05) in myocytes from patients with AF but only by approximately 18% (p < 0.05) in myocytes from SR atria. Proarrhythmic activity was elicited in atrial trabeculae exposed to high [Ca(2+)](o) or isoprenaline, which was significantly reversed by Ran (by 83% and 100%, respectively). Increasing pacing rates from 0.5 to 3.0 Hz led to an increase in diastolic tension that could be significantly decreased by Ran in atria from SR and AF patients. Na(+) channels may contribute to arrhythmias and contractile remodeling in AF. Inhibition of I(Na) with Ran had antiarrhythmic effects and improved diastolic function.
PMID:20488304 Sossalla S et al; J Am Coll Cardiol 55 (21): 2330-42 (2010)
For more Mechanism of Action (Complete) data for Ranolazine (11 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Not Available
Application : Co-Processed Excipients
Excipient Details : Standard Direct Tabletting Or Roller Compaction
Dosage Form : Tablet
Grade : Not Available
Application : Co-Processed Excipients
Excipient Details : Direct Compression
Pharmacopoeia Ref : NF/EP/JP
Technical Specs : Not Available
Ingredient(s) : Spray Dried Monohydrate Lactose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Film Forming Agent, Wet/Dry Granulation- Binder,Thickening & Suspension Agent, Non-Gelatin Capsule Manufacturing & Enteric Film Coating Systems
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Not Available
Application : Controlled & Modified Release
Excipient Details : Sustained Release Tablet Matrix
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Not Available
Application : Controlled & Modified Release
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Polydextrose Sugar
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Enteric Coatings
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : EthylCellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Customized per requirements
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
68
PharmaCompass offers a list of Ranolazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ranolazine manufacturer or Ranolazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ranolazine manufacturer or Ranolazine supplier.
PharmaCompass also assists you with knowing the Ranolazine API Price utilized in the formulation of products. Ranolazine API Price is not always fixed or binding as the Ranolazine Price is obtained through a variety of data sources. The Ranolazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ranolazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ranolazine, including repackagers and relabelers. The FDA regulates Ranolazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ranolazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ranolazine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ranolazine supplier is an individual or a company that provides Ranolazine active pharmaceutical ingredient (API) or Ranolazine finished formulations upon request. The Ranolazine suppliers may include Ranolazine API manufacturers, exporters, distributors and traders.
click here to find a list of Ranolazine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ranolazine DMF (Drug Master File) is a document detailing the whole manufacturing process of Ranolazine active pharmaceutical ingredient (API) in detail. Different forms of Ranolazine DMFs exist exist since differing nations have different regulations, such as Ranolazine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ranolazine DMF submitted to regulatory agencies in the US is known as a USDMF. Ranolazine USDMF includes data on Ranolazine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ranolazine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ranolazine suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Ranolazine Drug Master File in Korea (Ranolazine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Ranolazine. The MFDS reviews the Ranolazine KDMF as part of the drug registration process and uses the information provided in the Ranolazine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Ranolazine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Ranolazine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Ranolazine suppliers with KDMF on PharmaCompass.
A Ranolazine written confirmation (Ranolazine WC) is an official document issued by a regulatory agency to a Ranolazine manufacturer, verifying that the manufacturing facility of a Ranolazine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ranolazine APIs or Ranolazine finished pharmaceutical products to another nation, regulatory agencies frequently require a Ranolazine WC (written confirmation) as part of the regulatory process.
click here to find a list of Ranolazine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ranolazine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ranolazine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ranolazine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ranolazine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ranolazine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ranolazine suppliers with NDC on PharmaCompass.
Ranolazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ranolazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ranolazine GMP manufacturer or Ranolazine GMP API supplier for your needs.
A Ranolazine CoA (Certificate of Analysis) is a formal document that attests to Ranolazine's compliance with Ranolazine specifications and serves as a tool for batch-level quality control.
Ranolazine CoA mostly includes findings from lab analyses of a specific batch. For each Ranolazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ranolazine may be tested according to a variety of international standards, such as European Pharmacopoeia (Ranolazine EP), Ranolazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ranolazine USP).
