
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
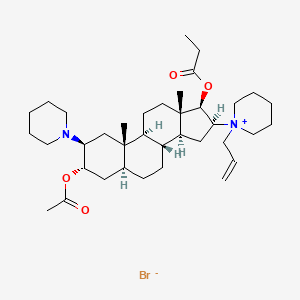

1. Org 9487
2. Org-9487
3. Rapacuronium
4. Raplon
1. Raplon
2. 156137-99-4
3. Org 9487
4. Org-9487
5. 156137-99-4 (bromide)
6. 65q4qdg4kc
7. 1-((2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-3-acetoxy-10,13-dimethyl-2-(piperidin-1-yl)-17-(propionyloxy)hexadecahydro-1h-cyclopenta[a]phenanthren-16-yl)-1-allylpiperidin-1-ium Bromide
8. Raplon (tn)
9. Ncgc00183839-01
10. Unii-65q4qdg4kc
11. Rapacuronium Bromide [usan:inn:ban]
12. Dsstox_cid_28749
13. Dsstox_rid_83018
14. Dsstox_gsid_48823
15. Schembl41132
16. 1-(3alpha-acetoxy-2beta-(1-piperidinyl)-17beta-(propionyloxy)-5alpha-androsta-16beta-yl)-1-allylpiperidinium Bromide
17. Chembl1200549
18. Dtxsid7048823
19. Rapacuronium Bromide [mi]
20. Rapacuronium Bromide (usan/inn)
21. Rapacuronium Bromide [inn]
22. Rapacuronium Bromide [usan]
23. Rapacuronium Bromide [vandf]
24. Tox21_113304
25. Rapacuronium Bromide [mart.]
26. Rapacuronium Bromide [who-dd]
27. Cs-6564
28. 1-allyl-1-(3alpha,17beta-dihydroxy-2beta-piperidino-5alpha-androstan-16beta-yl)piperidinium Bromide, 3-acetate 17-propionate
29. Hy-16423
30. Piperidinium, 1-((2beta,3alpha,5alpha,16beta,17beta)-3-(acetyloxy)-17-(1-oxopropoxy)-2-(1-piperidinyl)androstan-16-yl)-1-(2-propenyl)-, Bromide
31. Rapacuronium Bromide [orange Book]
32. Cas-156137-99-4
33. D05703
34. Q3419354
35. [(2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-3-acetyloxy-10,13-dimethyl-2-piperidin-1-yl-16-(1-prop-2-enylpiperidin-1-ium-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl] Propanoate;bromide
36. 1-allyl-1-(3.alpha.,17.beta.-dihydroxy-2.beta.-piperidino-5.alpha.-androstan-16.beta.-yl)piperidinium Bromide, 3-acetate 17-propionate
| Molecular Weight | 677.8 g/mol |
|---|---|
| Molecular Formula | C37H61BrN2O4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 676.38147 g/mol |
| Monoisotopic Mass | 676.38147 g/mol |
| Topological Polar Surface Area | 55.8 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1030 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Neuromuscular Nondepolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction without causing depolarization of the motor end plate. They prevent acetylcholine from triggering muscle contraction and are used as muscle relaxants during electroshock treatments, in convulsive states, and as anesthesia adjuvants. (See all compounds classified as Neuromuscular Nondepolarizing Agents.)
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?